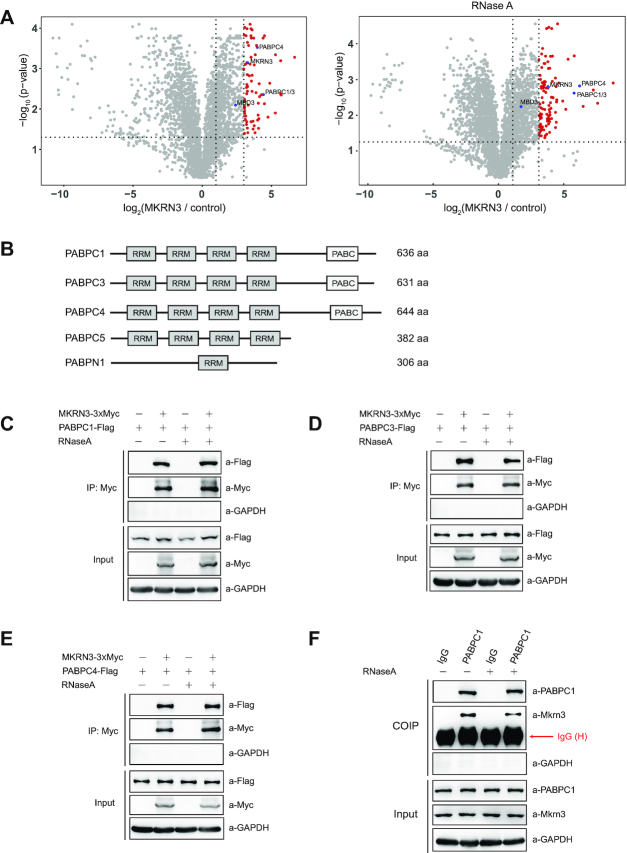
Figure 1.
MKRN3 forms complex with poly(A)-binding protein (PABP) family members. (A) Identification of MKRN3-interacting proteins through co-immunoprecipitation followed by mass spectrometry analysis. Volcano plots depicting the proteins enrichment from co-immunoprecipitation experiments, and the red circles represent proteins enriched >8-fold by MKRN3 compare to empty vectors. HEK293T cells were ectopically expressing 3xFlag-tagged MKRN3 or Flag tagged empty vectors. Forty-eight hours later, cells lysates treated with or without RNaseA (50 μg/ml), were incubated with anti-Flag affinity gels, and the co-immunoprecipitates were subjected to trypsin digestion followed by mass spectrometry analysis. MKRN3, PABP family members including PABPC1, PABPC3 and PABPC4, and previously reported MKRN3 interacting protein MBD3 indicated in blue colour. Three samples each group. (B) Domain structures of the PABP family members, including PABPN1, PABPC1, PABPC3 PABPC4 and PABPC5, which commonly share RNA-binding RRM (RNA recognition motif). PABC, poly(A)-binding protein C-terminal domain. (C–F) 3XMyc tagged MKRN3 protein was co-immunoprecipitated with Flag-tagged PABPC1 (C), PABPC3 (D) or PABPC4 (E) when co-expressed in HEK293T cells. Cell lysates treated with or without RNaseA (50 μg/ml) were subjected to co-immunoprecipitation assay using anti-Myc antibody, followed by immunoblotting with anti-Myc or anti-Flag antibody. (F) Endogenous Mkrn3 and PABPC1 formed a complex in the hypothalamus of wild-type mouse. Endogenous PABPC1 proteins of hypothalamic lysates from wild-type male mice at postnatal day 15 treated with or without RNaseA were immunoprecipitated using anti-PABPC1, followed by immunoblotting with indicated antibodies.