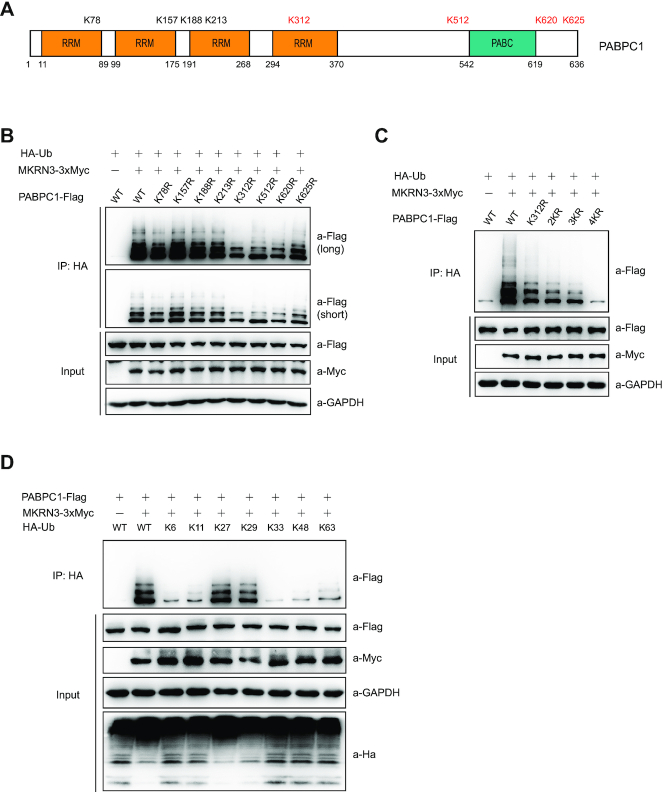
Figure 4.
MKRN3 mediates non-proteolytic ubiquitination of PABPC1 at multiple sites. (A) Schematic distribution of the eight sites (Lys residues) for MKRN3-mediated ubiquitination on human PABPC1 identified by mass spectrometry analysis. PABPC1 proteins were recovered from the in vitro ubiquitination assay and subjected to trypsin digestion, followed by mass spectrometry analysis to map the ubiquitination sites. (B) Four Lys residues (K312, K512, K620 and K625, shown red colour in A) were shown to be the major sites for MKRN3-mediated ubiquitination of PABPC1. Lysates of MKRN3−/-HEK293T cells ectopically expressing HA tagged Ub, 3XMyc tagged MKRN3, and Flag tagged PABPC1 or the indicated K-to-R mutants. Cell lysates were immunoprecipitated with anti-HA affinity gels, followed by immunoblotting analysis using anti-Flag to detect the ubiquitinated PABPC1 and other antibodies indicated. (C) Simultaneous four K-to-R mutation at the major ubiquitination sites almost totally abolished the ubiquitination of PABPC1 mediated by MKRN3. MKRN3−/-HEK293T cells were ectopically expressing HA tagged Ub, 3XMyc tagged MKRN3 and Flag-tagged PABPC1 or the mutants bearing the indicated K-to-R mutations. Cell lysates were immunoprecipitated with anti-HA affinity gels, followed by immunoblotting using anti-Flag to detect the ubiquitinated PABPC1. 2KR, K312–512R; 3KR, K312–512-620R; 4KR, K312–512-620–625R. (D) MKRN3 ubiquitinated PABPC1 with non-proteolytic K27 and k29 ubiquitin linkages. MKRN3−/–HEK293T cells were ectopically co-expressing 3xMyc-tagged MKRN3, Flag-tagged PABPC1 and HA tagged Ub (WT, or mutated at Lys6, Lys11, Lys27, Lys29, Lys33, Lys48 or Lys63 only) in the indicated combinations. Cell lysates were were immunoprecipitated with anti-HA affinity gels, followed by immunoblotting using anti-Flag to detect the ubiquitinated PABPC1 and other antibodies indicated.