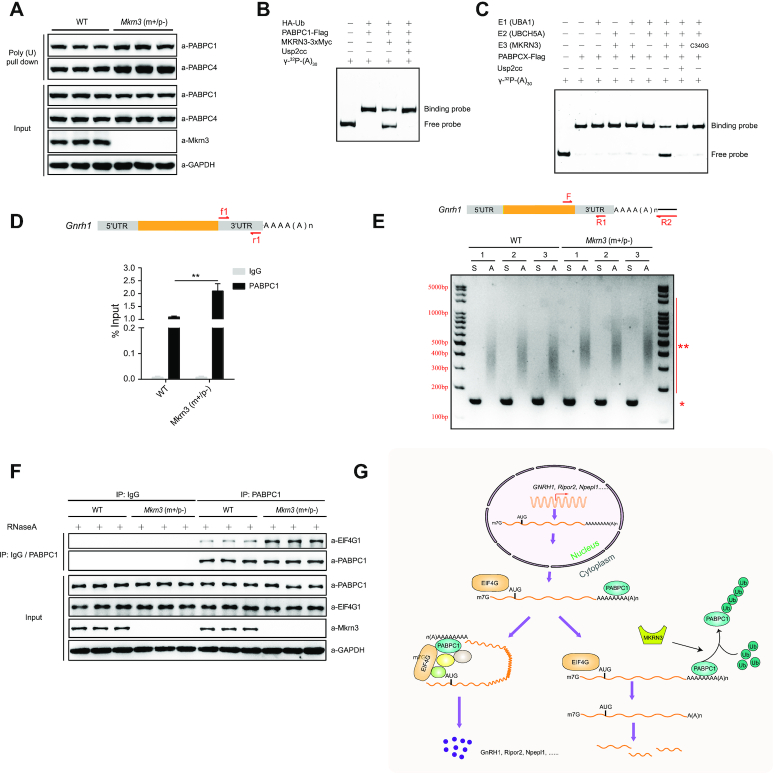
Figure 6.
The ubiquitination of PABPs by MKRN3 comprises its binding to the poly(A) of mRNA and the formation of translation initiation complex (TIC). (A) More PABPC1 and PABPC4 proteins were recovered with poly(A)-tailed mRNAs in the hypothalamus of Mkrn3m+/p- mice than those from age-matched wild-type littermates. The total ploy(A)-tailed mRNAs were recovered with poly (U) pull-down assay, in which lysates of hypothalamic tissues were precipitated using poly(U) agarose beads (see Materials and methods for details). The poly(A)-bound PABPC1 and PABPC4 were detected by immunoblotting using indicated antibodies, three mice were used in each group. (B) MKRN3 mediated the ubiquitination of PABPC1 compromised its binding to (A)30 RNA as detected by RNA EMSA. MKRN3−/− HEK293 cells were co-transfected with HA-tagged Ub, Flag-tagged PABPC1, and 3xMyc-tagged MKRN3 or not. 24 hours later, cell lysates were immunoprecipitated using anti-Flag affinity gels, treated with Usp2cc or not, and eluted with Flag peptides before subjected to RNA EMSA analysis (see Materials and methods for details). Usp2cc, the catalytic core of human deubiquitinase Usp2. (C) MKRN3-mediated ubiquitination compromised the affinity of PABPC1 to (A)30 RNA. EMSA assay was performed using (A)30 RNA probe that labeled with γ-32P. Recombinant PABPC1 was subjected to in vitro ubiquitination under varying indicated conditions, then immunoprecipitated with anti-Flag affinity gels before subjected to the EMSA assay. (D) Ablation of endogenous Mkrn3 promoted the binding of endogenous PABPC1 to Gnrh1 mRNA in the hypothalamus of Mkrn3m+/p− mice compared to that of wild-type ones. RNA immunoprecipitation assay was done with the hypothalamus of wild-type and Mkrn3m+/p− male mice at post-natal day 15, and PABPC1-bound mRNAs were enriched with anti-PABPC1 antibodies followed by qPCR assays using primers f1+r1. Data were presented as Mean ± SD, two-tailed unpaired t-test, n = 5. **P < 0.01, very significant difference. (E) The poly(A) tail-length of Gnrh1 mRNAs in the hypothalamus of Mkrn3m+/p− mice were longer than those in wild-type. Total RNAs of hypothalamic tissues were prepared from male mice at post-natal day 15, followed by addition of a limited number of guanosine and inosine residues to the 3′-ends of poly(A)-containing RNAs, to allow reverse transcription-PCR (RT-PCR) amplification using primer R2 (see Materials and methods for details). S, gene-specific amplicons to tell the abundance of Gnrh1 mRNA, using primers F+R1; A, poly(A)-tail-containing amplicons to tell the poly(A) tail-length of Gnrh1 mRNA, using primers F+R2.*, gene-specific bands; **, poly(A) tail-length bands, three samples in each group. (F) Ablation of endogenous Mkrn3 promoted PABPC1-EIF4G1 interaction in the hypothalamus of mice. Co-immunoprecipitation assay were performed with the hypothalamus of wild-type and Mkrn3m+/p− mice at post-natal day 15 using anti-IgG or anti-PABPC1 antibody, treated with RNaseA (50 μg/ml) or not, followed by immunoblotting with indicated antibodies, three samples in each group. (G) A model depicting how the MKRN3-PABPC1 axis controls the post-transcriptional switch of mammalian puberty initiation through regulating the stability and translation of mRNAs (including GNRH1) in hypothalamic cells.