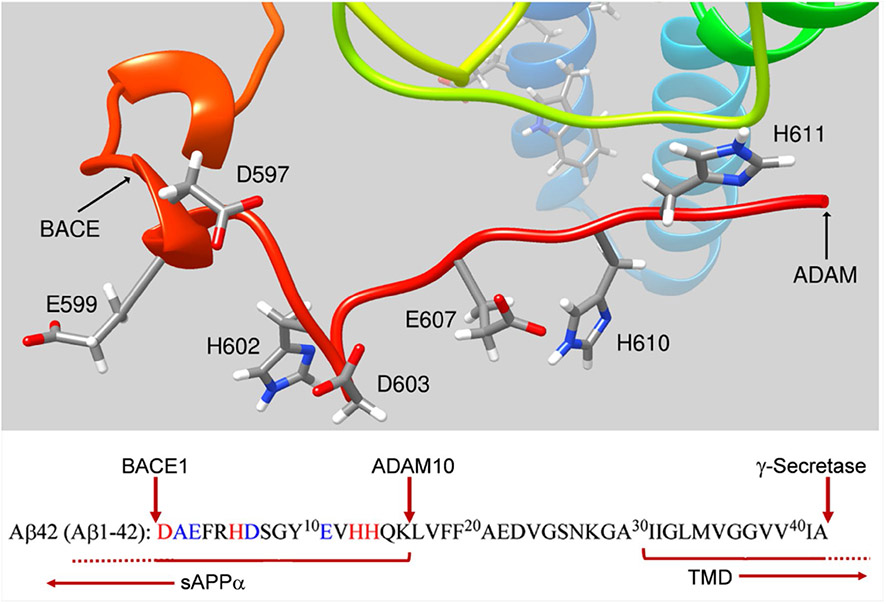
Fig. 3.
Retention of metal-binding epitopes in sAPPα. ADAM10/17 processing of APP cleaves the native APP at the carboxyl-terminal end of the well-characterized Aβ motif associated with metal binding (highlighted in red and blue and noted in the structure). The amyloidogenic processing pathway associated with BACE1 processing is indicated, also. In either pathway, membrane-associated secretase function ‘cleans up’ the residual transmembrane domain fragment releasing the C-terminal domain into the cytosol (Fig. 1). Reasonably, sAPPα should exhibit whatever metal binding has been attributed to Aβ. The residue numbering in the sequence reflects that used for Aβ, while the residue numbering in the structure is for sAPPα generated from APP695, the most abundant neuronal isoform