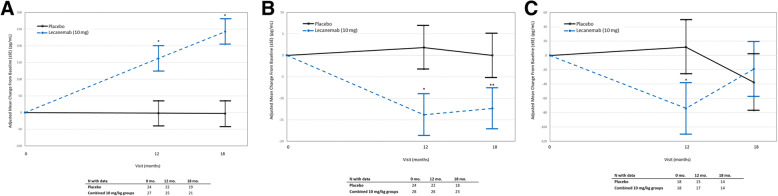
Fig. 5.
Change from baseline in CSF biomarker measures. a Change from baseline in CSF Aβ1–42 measures. The combined 10 mg/kg monthly and 10 mg/kg biweekly group is compared versus placebo. The number of subjects that were assessed at each time point are indicated in the table. The MMRM used treatment group, visit, clinical subgroup (MCI due to AD, Mild AD), the presence or absence of ongoing AD treatment at baseline, ApoE4 status (positive, negative), region, treatment group-by-visit interaction as factors, and baseline value as covariate. *P < 0.001. b Change from baseline in p-tau measures. The combined 10 mg/kg monthly and 10 mg/kg biweekly group is compared versus placebo. The number of subjects that were assessed at each time point are indicated in the table. The MMRM used treatment group, visit, clinical subgroup (MCI due to AD, Mild AD), the presence or absence of ongoing AD treatment at baseline, ApoE4 status (positive, negative), region, treatment group-by-visit interaction as factors, and baseline value as covariate. *P < 0.001, **P = 0.005. c Change from baseline in t-tau measures. The combined 10 mg/kg monthly and 10 mg/kg biweekly group is compared versus placebo. The number of subjects that were assessed at each time point are indicated in the table. The MMRM used treatment group, visit, clinical subgroup (MCI due to AD, Mild AD), the presence or absence of ongoing AD treatment at baseline, ApoE4 status (positive, negative), region, treatment group-by-visit interaction as factors, and baseline value as covariate. *P = 0.029