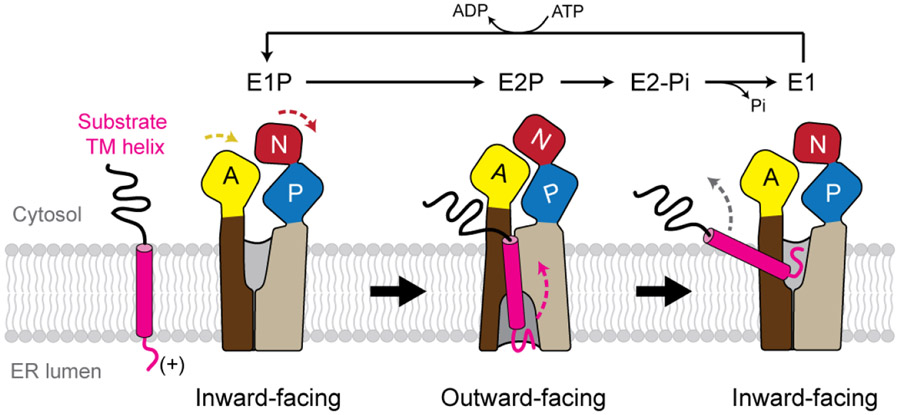
Fig. 5. Proposed model for TM dislocation by the P5A-ATPase.
The P5A-ATPase likely alternates between mainly two conformations, inward-open E1 and outward-open E2 states. During the E1P-to-E2P transition, the N domain rotates (red), and the A domain (yellow) moves closer to the N-P interface, causing conversion of the inward-open substrate-binding pocket of the transmembrane domain (brown and tan) to the outward-open conformation. In this model, a substrate TM helix (magenta) with a short, preferentially positively charged luminal segment would bind to the outward-open pocket and the E2P-to-E1 transition would flip the TM by a switch from the outward-open to inward-open conformation. For simplicity, some intermediate steps of the Post-Albers cycle are not shown.