Figure 1:
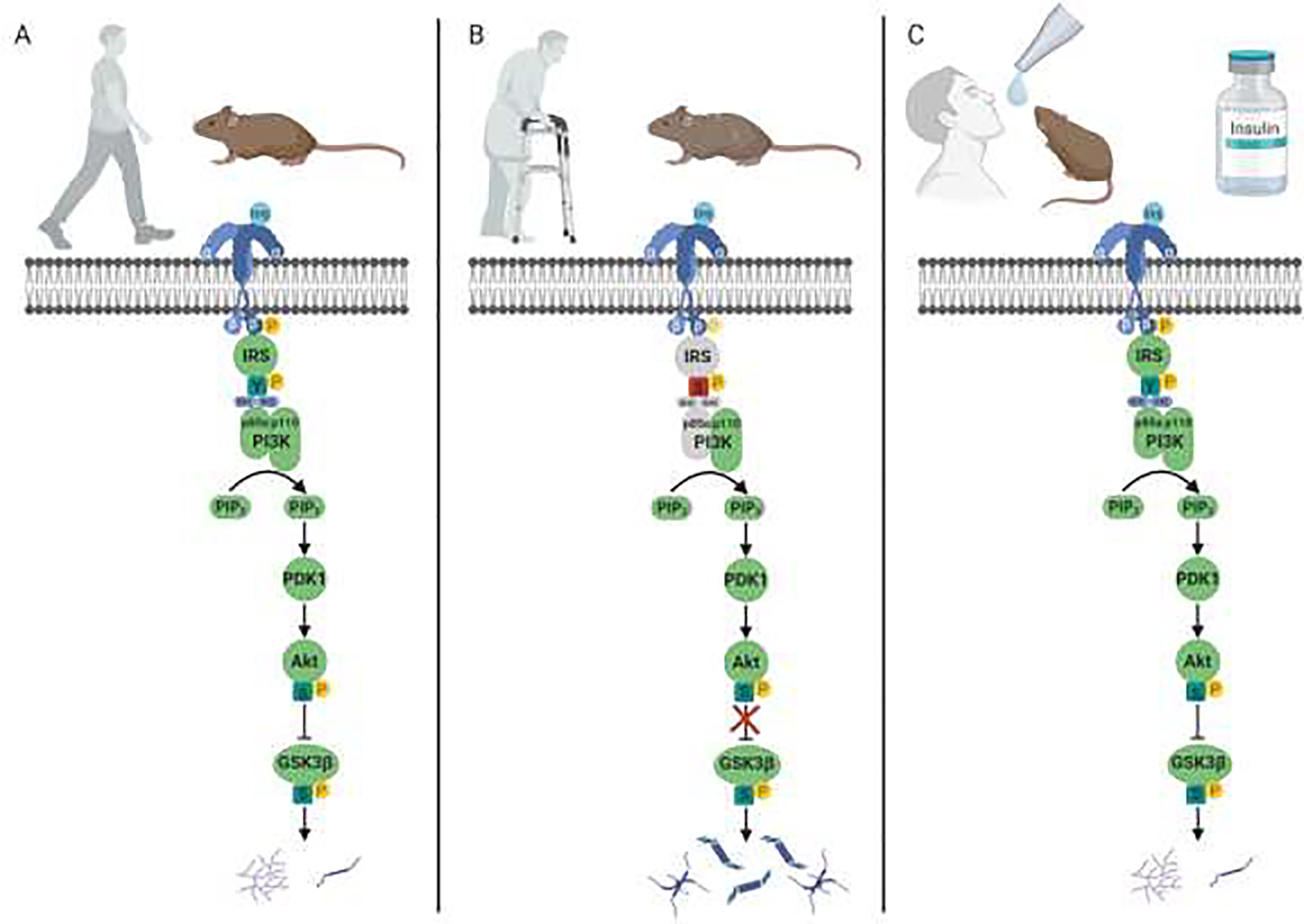
Intranasal insulin administration may treat age-related cognitive decline via restoration of brain insulin signaling to overcome insulin resistance. A) In the healthy young adult brain, insulin binds to the insulin receptor, activating the intrinsic tyrosine kinase activity and initiating a phosphorylation cascade. The activated insulin receptor phosphorylates insulin receptor substrate (IRS) proteins at tyrosine residues (Y), which bind to Src homology 2 (SH2) domains of the p85α subunit of phosphoinositide-3 kinase (PI3K). This catalyzes the formation of phosphatidylinositol-3,4,5-trisphosphate (PIP3). This then stimulates phosphoinositide-dependent kinase (PDK1), which phosphorylates and activates Akt at Serine (S) 473. Akt phosphorylates, and thus inhibits, glycogen synthase kinase 3β (GSK3β) at Serine (S) 9, thereby stimulating glycogen synthesis (purple) and the phosphorylation of tau protein (dark blue). B) A reduction in tyrosine kinase and levels of insulin receptor subtrate proteins, as shown here in gray, has been shown with aging and Alzheimer’s disease Post-mortem analysis of brains of patients with Alzheimer’s disease has also revealed increased phosphorylation at serine residues on IRS-1. Unlike tyrosine phosphorylation of IRS-1, phosphorylation at serine residues (red S) can inhibit IRS-1 activity. A decrease in IRS-1 binding to the p85α subunit of PI3K (shown in gray) has also been demonstrated in AD brains. Finally, dysregulated insulin signaling may lead to the overactivation of GSK3β, thus inhibiting glycogen synthesis and promoting the hyperphosphorylation of tau activity and aggregating into neurofibrillary tangles. C) The intranasal administration of insulin may increase insulin receptor and IRS-1 activation to enhance PI3K/Akt activity, ultimately restoring insulin signaling in the brain to overcome age-related cognitive decline.
