Abstract
Gastrointestinal stromal tumors (GISTs) are rare tumors of the gastrointestinal (GI) tract yet represent the most common GI sarcomas. Most GISTs are driven by activating mutations of the KIT and/or PDGFRA genes. Prior to the development of tyrosine kinase inhibitors (TKIs), GISTs were associated with a poor prognosis because conventional cytotoxic chemotherapy was relatively ineffective. However, TKIs that inhibit the most common driver mutations in KIT or PDGFRA have revolutionized the treatment of GISTs over the past two decades. Notwithstanding, ongoing management challenges relate to the development of secondary mutations in these genes, resulting in tumor progression. Due to both the intra- and inter-patient heterogeneity of these secondary mutations in GISTs, optimal treatment requires an agent that blocks as many mutant genes as possible. Ripretinib – a novel switch-control TKI – inhibits many of the most common primary and secondary activating KIT and PDGFRA mutants involved in GIST progression through a dual mechanism of action. In the pivotal INVICTUS phase III trial, patients with advanced GIST that had progressed on at least imatinib, sunitinib, and regorafenib and who received ripretinib experienced significantly longer progression-free survival (primary endpoint) as well as prolongation of overall survival, compared with those receiving placebo. Treatment with ripretinib was associated with durable improvements in quality-of-life indices and a manageable toxicity profile. The most frequent side effects were common to the class of TKIs used in the management of GIST. These results led to the approval of ripretinib for treatment of advanced GIST in adults who have received three or more TKIs, including imatinib. Ripretinib is also under investigation in the second-line treatment of advanced GIST in a phase III trial (INTRIGUE) comparing ripretinib with sunitinib in patients with advanced GIST after treatment with imatinib.
Plain language summary
Use of ripretinib for the treatment of gastrointestinal stromal tumors (GISTs)
Gastrointestinal stromal tumors (GISTs) are a rare type of tumor most commonly located in the stomach and small intestine but can develop anywhere throughout the gastrointestinal tract. The symptoms of GISTs vary in extent depending on location of the primary tumor and include a feeling of fullness, abdominal pain, intestinal bleeding, and fatigue. Since these symptoms are nonspecific, making a diagnosis can be challenging. Most GISTs carry initial mutations in genes that control specific enzymes called tyrosine kinases. Historically, treatment of GISTs was limited because traditional chemotherapy is ineffective against these tumors. However, with the introduction of drugs that inhibit tyrosine kinases [i.e., tyrosine kinase inhibitors (TKIs)], survival has been extended substantially. However, many GISTs go on to develop secondary mutations that render them resistant to a given TKI. Prior to the approval of ripretinib, four TKIs were available for the treatment of GIST: imatinib; sunitinib; regorafenib; and, recently, avapritinib. Each drug is used until resistance develops or patients are unable to tolerate the side effects of treatment, after which the next drug is started. Ripretinib was recently approved by the FDA as the fourth drug in the usual treatment sequence recommended for patients with advanced GIST who have progressed (or are treatment intolerant) after receiving three or more TKIs, including imatinib. Approval of ripretinib was based on the results of the INVICTUS trial, which demonstrated that the drug significantly improves the time patients have without progression of the disease or death compared with placebo. The most common side effects related to ripretinib were hair loss, muscle pain, nausea, fatigue, hand-foot syndrome, and diarrhea, although most events were not very severe. Ripretinib is being further studied as the second TKI used in patients with GIST who have progressed on or could not tolerate first-line treatment with imatinib.
Keywords: gastrointestinal stromal tumor, INTRIGUE, INVICTUS, ripretinib, tyrosine kinase
Introduction
Gastrointestinal stromal tumors (GISTs) are the most common soft tissue sarcomas of the gastrointestinal (GI) tract.1 GISTs are considered rare tumors, though the true incidence is unknown and is confounded by the prevalence of incidental microGISTs.2 Most epidemiological studies report an incidence between 10 and 15 cases per million individuals, but there has been an increased incidence over time with greater recognition of this entity3; this may reflect an increased identification of tumors rather than a true increased incidence. Men and women are equally affected, and GISTs are rare in patients under the age of 18 (1−2% of patients).3,4 The median age at presentation ranges from the mid-50s to the mid-60s.3,4 GISTs can occur in any part of the GI tract, but they are seen most commonly in the stomach (55−60%) and small intestine (30%), and less frequently in the duodenum (4−5%), rectum (2−4%), colon/appendix (1−2%), and esophagus (<1%).1,3,5
There is a wide range of modes of presentation, from asymptomatic to metastatic at diagnosis, depending in part on the anatomic organ in which the tumor originates.1,3 Symptoms can be nonspecific and include early satiety, abdominal pain or swelling, dyspepsia, vomiting, GI bleeding, and fatigue related to anemia. Less common symptoms include intraperitoneal hemorrhage and GI obstruction.6,7
The large majority of GISTs (95%) express KIT (CD117), usually with a diffuse cytoplasmic staining pattern.1,5 Most GISTs have a mutation in the KIT proto-oncogene receptor tyrosine kinase (KIT; 65–80%) or in the related platelet-derived growth factor-α (PDGFRA) receptor tyrosine kinase (5–10%), with approximately 10–15% of tumors having no mutation in either of these two genes; referred to as wild type for KIT or PDGFRA mutations.1,5 The most common KIT mutation involves exon 11, located in the juxtamembrane domain (70% of GISTs) of KIT, while mutations in the extracellular domain of KIT involving exon 9 are observed in 9−20% of tumors.1,5 Other common, but less definitive, markers of GIST include expression of the CD34 antigen (70−90% of tumors), actin (20−30%), S-100 (8−10%), and desmin (2−4%).1,5
Surgery is the standard treatment for localized and resectable GIST (⩾2 cm), with a goal of complete removal.1,2,5 Gastric tumors generally have a more favorable prognosis compared with intestinal GISTs.8 The most important prognostic factors are tumor size, tumor site, and mitotic rate, although these features alone are not sufficient to predict the metastatic potential of GISTs.1 Among gastric GISTs, those that are ⩽10 cm and have five or fewer mitoses per 50 high-power fields (HPFs; 50 HPFs = total area of 5 mm2) are considered at low risk for metastasis, while those >5 cm and with more than five mitoses per 50 HPFs are high risk.1 For intestinal GISTs, all tumors ⩾5 cm are considered at least moderate risk, while those with more than five mitoses per 50 HPFs are considered at high risk for metastases. Low-risk intestinal GISTs are those ⩽5 cm in size with up to five mitoses per 50 HPFs.1,8 Tumor genotype can also influence prognosis. In gastric GISTs, KIT exon 9 duplication and KIT exon 11 deletions have been shown to be associated with poorer disease-free survival, while PDGFRA exon 18 mutations were associated with better prognosis.9
Preoperative treatment with the tyrosine kinase inhibitor (TKI) imatinib should be considered on an individual basis for patients with potentially resectable disease, but for whom complete resection with negative margins is likely to create a significant risk of substantial morbidity.1 Whether or not patients receive preoperative imatinib, patients with high risk of recurrence should receive postoperative imatinib for 3 years following resection of the primary GIST.1,2 For those patients with intermediate-risk GISTs, the benefits of adjuvant therapy (following resection) are less clear cut, as studies thus far have not identified a definitive increase in overall survival (OS) with such an approach; hence, this is often a decision taken by each patient after discussion about the risk/benefit ratio of treatment.5
Conventional cytotoxic chemotherapy is not effective for the treatment of GISTs and, prior to the introduction of TKIs, the median survival of patients with advanced GIST was approximately 18 months.5,10 However, with the advent of TKI therapy, the median survival for patients with advanced GIST has increased to 45–57 months.4 As such, for tumors that are unresectable, metastatic, or recur after adjuvant therapy, treatment with a TKI is recommended.1,2 While TKIs extend the expected survival of patients with GIST, secondary TKI-specific inactivating mutations may arise,5 resulting in tumor progression. To address secondary resistance to imatinib, several other TKIs with different mechanisms of action have been developed and approved for use in advanced or metastatic GIST. Ripretinib – a switch-control kinase inhibitor – was recently approved as a fourth-line-or-greater therapy for the treatment of advanced GIST in the United States (US), Canada, Australia, and Hong Kong.11–13
TKIs for treatment of advanced GIST
As mentioned above, before TKIs were available, treatment options for advanced or metastatic GIST were extremely limited. However, upon discovery that the large majority of GISTs are driven by activating mutations of KIT or PDGFRA, resulting in ligand-independent activation of the KIT receptor tyrosine kinase and unopposed growth of tumor cells, drugs targeting these mutations were rapidly introduced into the clinic with dramatic results.5 But with recognition of the importance of these mutations came the recognition that not all mutations were the same, with mutations in exon 9 in KIT conferring a somewhat poorer prognosis in advanced disease, whereas mutations in exon 18 of PDGFRA are associated with relatively indolent disease for patients with localized GIST.5 As such, mutation profiling is essential to determine the optimal course of therapy. Recurrence after individual TKI therapy is most often the result of secondary mutations in the same set of genes inhibited by the TKI being used, conferring resistance and necessitating the use of TKIs with different mechanisms of action.5
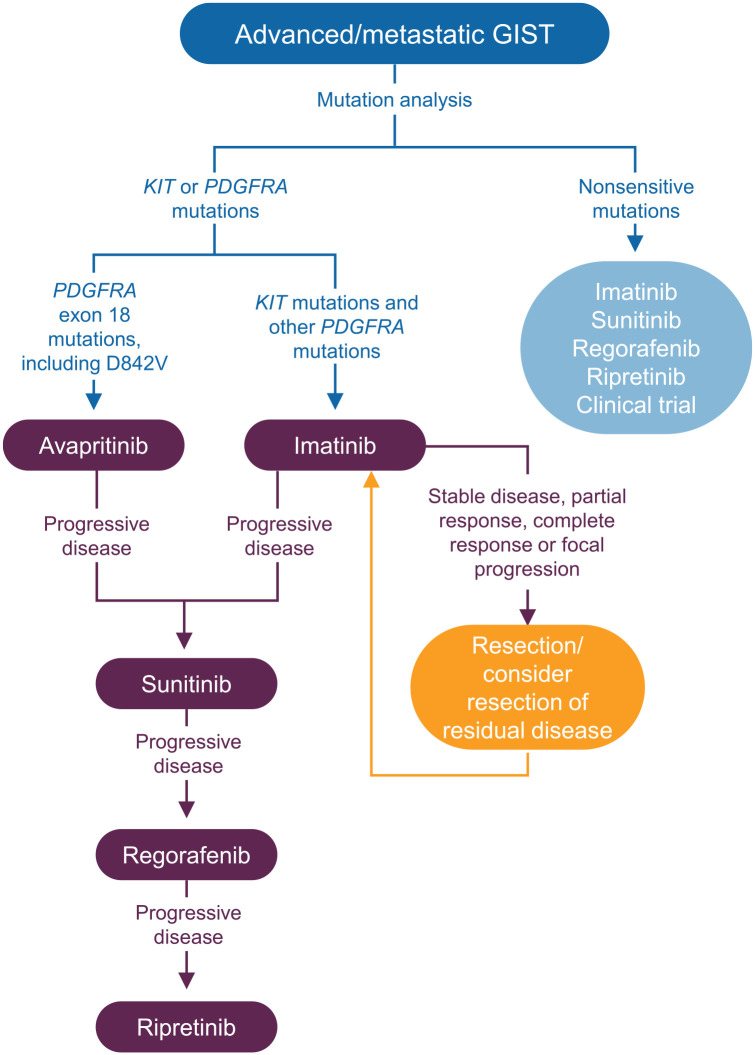
Imatinib is considered first-line therapy for patients with advanced, unresectable, or metastatic GIST or in those for whom surgery for localized GIST poses a risk of significant postoperative or longer-term functional complications (Figure 1).1,2 Several studies have demonstrated that imatinib produces sustained responses in patients with advanced or metastatic GIST.14–16 However, a notable exception are metastatic GISTs with primary mutations in the PDGFRA exon 18 gene (6% of the overall GIST population), particularly the most common subtype of activating mutation in the PDGFRA gene known as the PDGFRA D842V mutation. Tumors carrying this specific mutation in the PDGFRA exon 18 gene do not respond to imatinib; as a result, avapritinib is recommended for first-line therapy for this small subset of the patient population.1 However, for the vast majority of patients with GIST carrying a mutation in KIT, data support the use of sunitinib as the preferred second-line therapy in patients who develop progressive disease (PD) on imatinib.1 In this setting, sunitinib has demonstrated improved disease control, progression-free survival (PFS), and OS compared with placebo (although survival differences were not seen in landmark analysis performed after longer follow up).17,18 In the third-line setting, regorafenib is recommended for those with PD after imatinib and sunitinib (or intolerance to these medications).1 For these patients, regorafenib similarly demonstrated improved disease control and increased PFS; however, there were no significant improvements in survival after crossover.19 For some patients, these therapies in the second or third line are not very tolerable or are accompanied by significant adverse side effects, resulting in dose reductions, interruptions, or discontinuation.18,19 Furthermore, due to the extensive heterogeneity of the disease, there existed an unmet need for therapies designed to show activity against a broad spectrum of mutations.
Figure 1.
Treatment algorithm for the use of approved TKIs for the management of advanced GIST based on ESMO and NCCN guidelines.1,2
ESMO, European Society for Molecular Oncology; GIST, gastrointestinal stromal tumor; NCCN, National Comprehensive Cancer Network; TKI, tyrosine kinase inhibitor.
Following US Food and Drug Administration (FDA) approval in May 2020, ripretinib is recommended for fourth-line therapy for unresectable or metastatic disease that has progressed after three or more TKIs, including imatinib.1,11 This article reviews the clinical pharmacology of ripretinib and the clinical efficacy and safety of the drug in patients with advanced GIST.
Ripretinib: mechanism of action
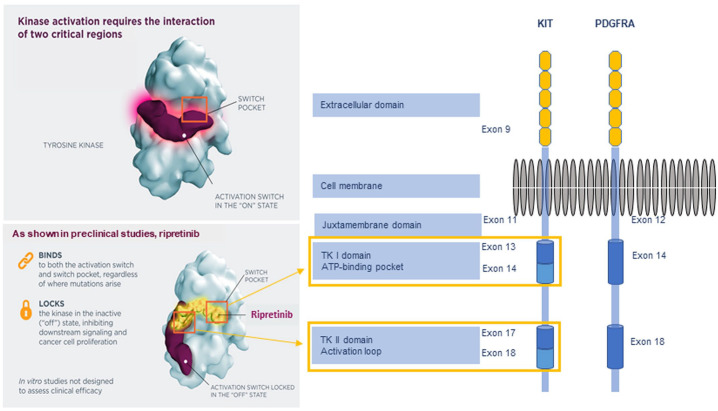
Ripretinib is a novel switch-control TKI that was specifically designed to inhibit both KIT and PDGFRA kinase signaling through a dual mechanism of action to secure the kinases in an inactive confirmation (Figure 2), thereby preventing the downstream signaling of survival and proliferation.20,21 KIT and PDGFRA are dual-switch kinases that have an auxiliary switch in the juxtamembrane domain encoded by KIT exon 11 or PDGFRA exon 12 and an activation loop in the kinase domain encoded by KIT exons 17 and 18 or PDGFRA exons 18 and 19.21,22 The switch pocket is the major controller of kinase activity, acting as an “on-off” switch for these two elements via phosphorylation of switch amino acids.21 Ripretinib binds to both the switch pocket region of tyrosine kinase and to the activation loop, locking the kinase in the inactivated state. The binding is both potent and durable.21
Figure 2.
KIT/PDGFRA structure and the mechanism of action of ripretinib.
ATP, adenosine triphosphate; TK, tyrosine kinase.
Since multiple types of secondary mutations can occur concurrently in TKI-resistant GIST, treatments that inhibit as many mutant genes as possible are highly desirable in order to block both the group of mutant genes that are present and those that may arise subsequently.21 Ripretinib has the potential to affect multiple secondary mutations. Enzymatic and GIST cell line studies demonstrated that ripretinib inhibits many of the most common primary and secondary KIT and PDGFRA mutants.21 Enzyme assays showed that ripretinib inhibits mutant KIT and PDGFRA activity at physiologic levels of adenosine triphosphate, including mutants resistant to imatinib, sunitinib, and regorafenib. This includes activity against the mutants KIT D816V, KIT D816H, KIT V654A, KIT T670I, and PDGFRA D842V.21 In GIST cell lines, ripretinib inhibited mutant KIT phosphorylation across a wide range of common primary and secondary mutants, including highly treatment-resistant mutants such as D816V.21 In these assays, ripretinib was effective against all imatinib- and sunitinib-resistant mutants and demonstrated greater activity than regorafenib in 18 of 37 mutants evaluated (3- to >50-fold greater potency) with similar potency (i.e., around one- to three-fold) in 17 mutants. Ripretinib was weaker than regorafenib against only two T670I secondary KIT mutants.21 In addition, no resistant clones to ripretinib were identified starting with either a KIT exon 11 mutant (V560D) or a KIT exon 17 mutant (D816V) in saturation mutagenesis assays.21
Efficacy and safety of ripretinib in GIST
Phase I trial
A phase I trial [ClinicalTrials.gov identifier: NCT02571036] of dose escalation with a subsequent expansion phase at the recommended phase II dose was conducted in patients with advanced GIST and other advanced malignancies who were intolerant to or experienced progression on more than one line of systematic therapy.23 Of 258 patients enrolled, 184 had GIST; of these, 68 patients were included in the dose-escalation phase and 190 in the expansion phase. In this study, patients received ripretinib 20–200 mg twice daily or 100–250 mg once daily in 28-day cycles until disease progression, unacceptable toxicity, or withdrawal of consent.
Less than 33% of patients experienced a dose-limiting toxicity, thus, no maximum tolerated dose was reached. Based on exposure-response analyses suggesting that >90% of patients receiving 150 mg once daily would achieve exposures of ripretinib and its active metabolite (DP-5439) that would produce >90% KIT inhibition, the 150-mg once-daily dose was selected for the phase II expansion portion of the trial.23
Among patients who received ripretinib 150 mg once daily in either the dose-escalation or expansion phase, the overall objective response rate (ORR; based on investigator assessment) was 11.3%, ranging from 7.2% (fourth-line) to 19.4% (second-line).23 Overall median PFS was 5.6 months, ranging from 5.5 months in fourth-line therapy to 10.7 months in second-line therapy. These results provided support for the further evaluation of ripretinib in the treatment of advanced GIST, including the phase III INVICTUS trial.
Phase III trial (INVICTUS)
The efficacy of ripretinib for treatment of patients with advanced GIST as fourth-line-or-greater therapy was established by the phase III, randomized, double-blind, placebo-controlled INVICTUS trial [ClinicalTrials.gov identifier: NCT03353753],20 which was conducted in 29 hospitals in 12 countries in North America, Europe, Australia, and Asia. Patients aged ⩾18 years with a diagnosis of GIST must have progressed on at least imatinib, sunitinib, and regorafenib, or to have had documented intolerance to any of these treatments despite dose modifications.
Enrolled patients were randomized 2:1 to receive ripretinib 150 mg orally or matching placebo once daily, both with best supportive care, until they developed PD, experienced intolerable adverse events (AEs), or withdrew consent. Upon disease progression, patients receiving placebo could cross over to ripretinib 150 mg once daily or discontinue the study, while those in the ripretinib group could dose escalate to 150 mg twice daily, continue at 150 mg once daily if showing clinical benefit, or discontinue therapy. Patients who crossed over from placebo to ripretinib and had further progression had the option of dose escalating to 150 mg twice daily. Dose interruptions and dose reductions were allowed at the discretion of the investigator.20
The primary efficacy endpoint was PFS according to modified RECIST 1.1 criteria and as assessed by a blinded independent central review. The key secondary endpoint was the ORR, defined as complete or partial response. Other secondary endpoints included OS, time to progression, time to best response, quality of life (QoL), and safety.20 The primary analyses data cutoff was 31 May 2019; additional longer-term analyses with a cutoff of 9 March 2020, were reported and are included below.
A total of 129 eligible patients were randomized to either ripretinib (n = 85) or placebo (n = 44).20 Demographic characteristics were generally similar between groups with median ages of 59 and 65 years, respectively, in ripretinib- and placebo-treated patients. In the ripretinib group, 64% had received three prior therapies and 36% had received 4−7 prior therapies; these proportions were 61% and 39%, respectively, in the placebo group. The primary tumor sites were gastric (47% versus 41%), jejunum or ileum (24% versus 18%), and duodenum (2% versus 18%) in the intervention versus placebo groups, respectively.20
Primary analyses
At the data cutoff for the primary analysis— 31 May 2019—the primary efficacy endpoint of median PFS was 6.3 months [95% confidence interval (CI) 4.6, 6.9] in the ripretinib group compared with 1.0 month (95% CI 0.9, 1.7) for the placebo group, resulting in a hazard ratio (HR) of 0.15 (95% CI 0.09, 0.25; p < 0.0001).20 Six-month PFS rates were 51% (39.4, 61.4) and 3.2% (0.2, 13.8), respectively, for the ripretinib and placebo groups.20 An objective response was observed in 8 of 85 (9.4%) patients receiving ripretinib; all were partial responses. No patient receiving placebo had a confirmed objective response. The median time to best response was 1.9 months (IQR 1.0, 2.7) in the ripretinib group and the median times to progression were 6.4 months (95% CI 4.6, 8.4) and 1.0 month (95% CI 0.9, 1.7), respectively, in the ripretinib and placebo groups.20
Median OS was 15.1 months (95% CI 12.3, 15.1) for patients receiving ripretinib and 6.6 months (95% CI 4.1, 11.6) for patients in the placebo arm with an HR of 0.36 (95% CI 0.21, 0.62).20 The 6- and 12-month survival rates were 84.3% (95% CI 74.5, 90.6) and 65.4% (95% CI 51.6, 76.1), respectively, in the ripretinib group; and 55.9% (95% CI 39.9, 69.2) and 25.9% (95% CI 7.2, 49.9), respectively, in the placebo group. However, since testing of the endpoints was hierarchical, there was no formal statistical assessment of OS.20 In patients originally assigned to placebo and then crossed over to ripretinib, median PFS and OS were 4.6 and 11.6 months, respectively,24 suggesting that patients achieved disease control after crossing over to ripretinib despite delayed initiation of treatment. However, since these findings were slightly lower than those for patients who initially received ripretinib, the maximum benefit may be achieved when the drug is used immediately after failure of prior therapy in patients with advanced GIST requiring therapy in the fourth-line-or-higher setting.24
During the INVICTUS trial, most AEs were Grade 1/2 in severity.20 The most common treatment-related AEs of any grade in the ripretinib group were alopecia (all Grade 1/2), myalgia, nausea, fatigue, palmar-plantar erythrodysesthesia (i.e., hand-foot syndrome; all Grade 1/2), and diarrhea. The most common treatment-emergent Grade 3/4 events were lipase increase (5%), hypertension (4%), fatigue (2%), and hypophosphatemia (2%). Eight (9%) patients receiving ripretinib experienced treatment-related serious AEs compared with 7% of placebo-treated patients.20 Serious events in the ripretinib group included anemia, cardiac failure, death of unknown cause, dyspnea, fecaloma, gastro-esophageal reflux disease, hyperkalemia, hypophosphatemia, nausea, and upper GI hemorrhage (one event each with some patients experiencing more than one serious event); 6% of patients receiving ripretinib had dose reductions because of treatment-related AEs and 5% of patients discontinued therapy related to AEs. The corresponding values for the placebo group were 2% and 2%, respectively. There was one treatment-related death in each group.20
A longitudinal analysis of alopecia and palmar-plantar erythrodysesthesia in the INVICTUS trial found that these events did not worsen over time.25 Further, patients with and without alopecia and palmar-plantar erythrodysesthesia had similar QoL scores that were generally stable over time. It is recommended that the dose of ripretinib be reduced to 100 mg once daily for clinically meaningful AEs.11 There are also specific dosage modifications in the package labeling for Grade ⩾2 palmar-plantar erythrodysesthesia syndrome, arthralgia, or myalgia and for Grade ⩾3 hypertension, left ventricular systolic dysfunction, and other AEs.11
The relative incidence of AEs with ripretinib compared with other TKIs is summarized in Table 1. While it is impossible to make firm conclusions regarding differences between agents because there are likely differences in patient characteristics in the studies of each agent, there are some apparent trends regarding AEs. There appeared to be differences in the incidence of palmar-plantar erythrodysesthesia (highest with regorafenib), nausea (highest with imatinib), skin discoloration (highest with sunitinib), and alopecia (highest with ripretinib).15,17,19,20 In addition, sunitinib appears to be associated with a higher risk of hematologic AEs (leucopenia, neutropenia, lymphopenia, thrombocytopenia).17 The majority of events for all agents were Grade 1/2 in severity (i.e., <5% for all events) with the exception of two events for regorafenib: hand-foot syndrome (20% Grade 3/4) and hypertension (23% Grade 3/4).19
Table 1.
Treatment-related AEs (⩾20%) reported in pivotal phase III trials of TKIs approved for the treatment of GIST.
| Adverse event (% of patients) | Imatinib 400 mg (N = 73)15 | Sunitinib (N = 202)17 | Regorafenib (N = 132)19 | Ripretinib (N = 85)20 | ||||
|---|---|---|---|---|---|---|---|---|
| Any grade | Grade 3/4 | Any grade | Grade 3/4 | Any grade | Grade 3/4 | Any grade | Grade 3/4 | |
| Hand-foot syndrome | NR | NR | 14 | 4 | 56 | 20 | 21 | 0 |
| Edema | 71 | 1 | NR | NR | NR | NR | NR | NR |
| Nausea | 51 | 1 | 24 | 1 | 16 | 1 | 26 | 1 |
| Diarrhea | 40 | 1 | 29 | 3 | 40 | 5 | 21 | 1 |
| Myalgia/musculoskeletal pain | 37 | 0 | NR | NR | 14 | 1 | 28 | 1 |
| Fatigue | 30 | 0 | 34 | 5 | 39 | 2 | 26 | 2 |
| Dermatitis/rash | 25 | 3 | 13 | 1 | 18 | 2 | NR | NR |
| Abdominal pain | 26 | 1 | NR | NR | NR | NR | NR | NR |
| Alopecia | NR | NR | NR | NR | 24 | 2 | 49 | 0 |
| Hypertension | NR | NR | 10 | 3 | 49 | 23 | 8 | 4 |
| Oral mucositis/mucosal inflammation | NR | NR | 12 | 0 | 38 | 2 | NR | NR |
| Skin discoloration | NR | NR | 25 | 0 | NR | NR | NR | NR |
AE, adverse event; GIST, gastrointestinal stromal tumor; NR, not reported; TKI, tyrosine kinase inhibitor.
Longer-term analyses
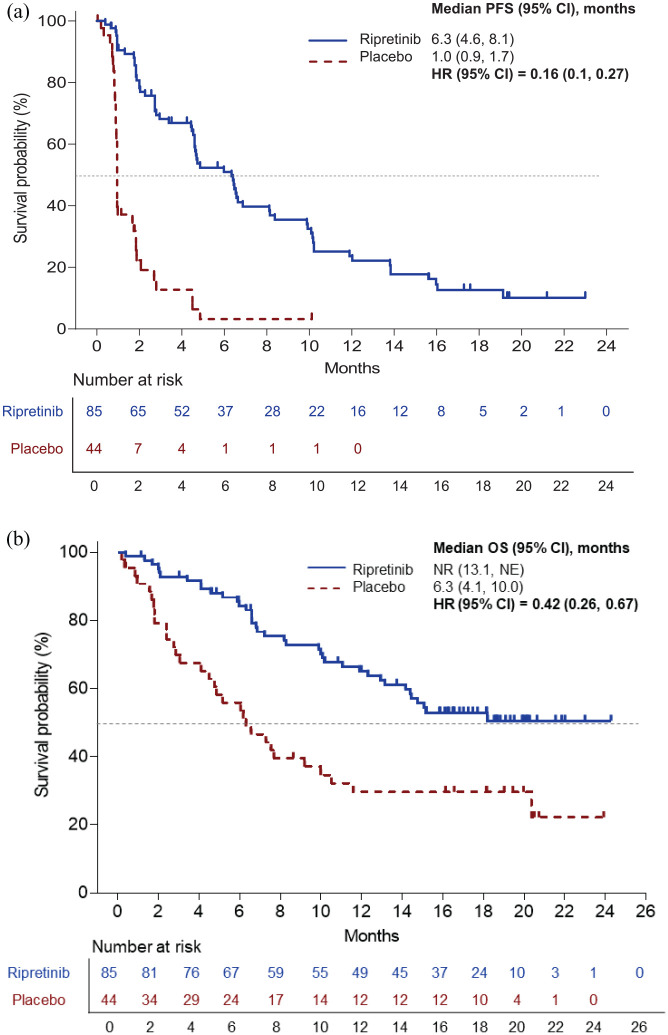
At the most recent data cut of 9 March 2020, after an additional 9 months of follow up, median PFS was 6.3 months (95% CI 4.6, 8.1) in the ripretinib group compared with 1.0 month (95% CI 0.9, 1.7) for the placebo group, resulting in an HR of 0.16 (95% CI 0.1, 0.27; Figure 3a). Among patients receiving ripretinib, the ORR was (11.8%; 10/85), all of which were partial responses. No confirmed objective responses occurred in the placebo group.26 Median duration of response in ripretinib-treated patients was 14.5 months (95% CI 3.7, not reached).26
Figure 3.
PFS (a) and OS (b) in patients receiving ripretinib or placebo in the INVICTUS trial.26,27
CI, confidence interval; HR, hazard ratio; NE, not estimable; NR, not reported; OS, overall survival; PFS, progression-free survival.
Median OS was not reached (95% CI 13.1, not reached) for patients receiving ripretinib and 6.3 months (95% CI 4.1, 10.0) for patients in the placebo group, with an HR of 0.42 (95% CI 0.26, 0.67; Figure 3b).26 In the ripretinib group, 6- and 12-month survival rates were 84.3% (95% CI 74.5, 90.6) and 65.1% (95% CI 53.6, 74.5), respectively; and 55.9% (95% CI 39.9, 69.2) and 26.7% (95% CI 16.8, 43.7), respectively, in the placebo group.
Grade 3/4 events reported most frequently in the 9 March 2020 data cut in ripretinib-treated patients were anemia (11%), hypertension (7%), abdominal pain (7%), lipase increase (5%), increase in blood alkaline phosphatase (5%), and hypophosphatemia (5%).27 By this later time point, dose reductions due to treatment-related AEs occurred in 8% of patients receiving ripretinib and 2% of patients receiving placebo; 8% and 2% of patients receiving ripretinib or placebo, respectively, discontinued therapy related to AEs.26 No additional deaths had occurred.26
Quality of life
In addition to safety and efficacy of a treatment, it is important to consider the effect of therapy on a patient’s QoL. In the INVICTUS trial, patient-reported outcomes were assessed using the Euro-Qol-5D (EQ-5D-5L) and the European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30).28 EQ-5D-5L is a self-assessed instrument that records health on a visual analog scale (VAS) ranging from 0 to 100 (worst to best imaginable state of health). The EORTC QLQ-C30 uses a 4-point scale (1 = not at all to 4 = very much) to assess physical function (e.g., strength, endurance, daily physical functioning) and role function (i.e., limitations during daily activities), and a 7-point scale (higher score is better) to assess overall health and overall QoL. Improvements are reflected as positive changes from baseline scores, while negative changes from baseline indicate declines in QoL. Patients receiving ripretinib reported improvements in health status on the EQ-5D-5L VAS (3.7) while those on placebo reported a decline in scores (−8.9). Similarly, patients receiving ripretinib compared with those receiving placebo reported greater improvements in physical function (1.6 versus −8.9) and role function (3.5 versus −17.1) along with higher perceptions of overall health (0.20 versus −0.78) and overall QoL (0.28 versus −0.76).28 The improvements in these QoL outcomes were maintained over time with stable scores out to cycle 10 of treatment. The differences between ripretinib and placebo met the threshold for clinically meaningful change.28,29
Discussion
Ripretinib, a novel switch-control TKI that inhibits both KIT and PDGFRA kinase signaling through dual mechanisms of action, inhibits many of the most common primary and secondary KIT and PDGFRA mutants involved in GIST progression. The superior efficacy of ripretinib versus placebo, on a background of best supportive care, along with its favorable safety profile and improvements in patients’ QoL, demonstrated in the INVICTUS trial, led to ripretinib’s approval for use as fourth-line-or-greater therapy for the treatment of patients with GIST. Continued longer-term follow up of patients enrolled in the INVICTUS trial suggest continued clinical benefit without additional safety concerns.
Imatinib rechallenge remains an option for patients with progressive disease no longer receiving benefit from their current TKI therapy.1 However, the clinical benefit of this approach is limited, based on data from a small, prospective, randomized, placebo-controlled study in 81 patients who experienced disease progression after two or more lines of TKI therapy; approximately 40% had received at least three prior TKI therapies.30 Median PFS in the imatinib rechallenge arm was modest – 1.8 months (95% CI 1.7, 3.6) – with no patient achieving an objective response.30 Thus, ripretinib is the preferred treatment regimen after patients have progressed on imatinib, sunitinib, and regorafenib.1
The efficacy and safety of ripretinib for second-line therapy of GIST is under investigation in the phase III INTRIGUE trial [ClinicalTrials.gov identifier: NCT03673501],31 which compares treatment with ripretinib versus sunitinib in patients with advanced GIST following failure of imatinib (due to progression or drug intolerance). The primary endpoint of the trial is PFS and key secondary endpoints include ORR and OS.31 The trial has met its target enrollment of 426 patients and top-line results are expected during the second half of 2021.32
In conclusion, the results of the INVICTUS trial have established ripretinib as the treatment of choice for patients who have unresectable or metastatic GIST that has progressed after treatment with three or more TKIs, including imatinib, with the exception of patients with a GIST carrying a mutation in exon 18 of PDGFRA. Ripretinib has a favorable toxicity profile and yielded improvements in patient-reported QoL compared with placebo. However, the ultimate place of ripretinib in metastatic or advanced GIST remains under investigation, including the phase III INTRIGUE study comparing ripretinib and sunitinib as second-line therapy in patients with advanced GIST after treatment with imatinib.
Footnotes
Conflict of interest statement: JZ reports the following potential conflicts of interest: honoraria from Pfizer, MSD, Merck, Specialized Therapeutics, Targovax, Halozyme, Gilead Sciences, Bayer, and Novella; institutional research grants from Bayer, Merck, Roche, BMS, Pfizer, AstraZeneca, Specialized Therapeutics, Baxalta/Shire, Lilly, Boehringer-Ingelheim, and MSD; travel support from Merck, AstraZeneca, MSD, Deciphera, and Sirtex; and owns stock in GW Pharmaceuticals, Aimmune, Vertex, Alnylam, BioMarin, Amarin, Freq Therapeutics, Global Blood Therapeutics, Gilead, Uniqure, Sangamo, Acceleron, Orphazyme, Moderna, Myokardia, Myovant, Concert Pharmaceuticals, Madrigal Pharmaceuticals, and Zogenix.
Funding: The author disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Medical writing and editorial support were provided by Bret Fulton and Meryl Gersh of AlphaBioCom, LLC, King of Prussia, PA, USA. This support was funded by Deciphera Pharmaceuticals, Inc., Waltham, MA, USA.
ORCID iD: John R. Zalcberg  https://orcid.org/0000-0002-6624-0782
https://orcid.org/0000-0002-6624-0782
References
- 1. National Comprehensive Cancer Network. Gastrointestinal Stromal Tumors (GISTs). Version 1.2021, https://www.nccn.org/professionals/physician_gls/default.aspx (accessed 18 November 2020).
- 2. Casali PG, Abecassis N, Aro HT, et al. Gastrointestinal stromal tumours: ESMO-EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018; 29: iv68–iv78. [DOI] [PubMed] [Google Scholar]
- 3. Soreide K, Sandvik OM, Soreide JA, et al. Global epidemiology of Gastrointestinal Stromal Tumours (GIST): a systematic review of population-based cohort studies. Cancer Epidemiol 2016; 40: 39–46. [DOI] [PubMed] [Google Scholar]
- 4. Call J, Walentas CD, Eickhoff JC, et al. Survival of gastrointestinal stromal tumor patients in the imatinib era: life raft group observational registry. BMC Cancer 2012; 12: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Poveda A, García Del Muro X, López-Guerrero JA, et al. GEIS guidelines for Gastrointestinal Sarcomas (GIST). Cancer Treat Rev 2017; 55: 107–119. [DOI] [PubMed] [Google Scholar]
- 6. Martin-Broto J, Martinez-Marin V, Serrano C, et al. Gastrointestinal Stromal Tumors (GISTs): SEAP-SEOM consensus on pathologic and molecular diagnosis. Clin Transl Oncol 2017; 19: 536–545. [DOI] [PubMed] [Google Scholar]
- 7. Lanke G, Lee JH. How best to manage gastrointestinal stromal tumor. World J Clin Oncol 2017; 8: 135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 2006; 23: 70–83. [DOI] [PubMed] [Google Scholar]
- 9. Wozniak A, Rutkowski P, Schöffski P, et al. Tumor genotype is an independent prognostic factor in primary gastrointestinal stromal tumors of gastric origin: a European multicenter analysis based on Contica GIST. Clin Cancer Res 2014; 20: 6105–6116. [DOI] [PubMed] [Google Scholar]
- 10. Bauer S, Joensuu H. Emerging agents for the treatment of advanced, imatinib-resistant gastrointestinal stromal tumors: current status and future directions. Drugs 2015; 75: 1323–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. QINLOCK. Prescribing information: Qinlock. Waltham, MA: Deciphera Pharmaceuticals, LLC, 2020. [Google Scholar]
- 12. Health Canada. QINLOCK™ product monograph, https://health-products.canada.ca/dpd-bdpp/dispatch-repartition.do;jsessionid=C58B3E156C26F4E7C8136E83FC5999CA (accessed 13 November 2020).
- 13. Australian Product Information. QINLOCKTM (ripretinib), https://www.tga.gov.au/product-information-0 (2020, accessed 13 November 2020).
- 14. Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol 2008; 26: 626–632. [DOI] [PubMed] [Google Scholar]
- 15. Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002; 347: 472–480. [DOI] [PubMed] [Google Scholar]
- 16. Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet 2004; 364: 1127–1134. [DOI] [PubMed] [Google Scholar]
- 17. Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet 2006; 368: 1329–1338. [DOI] [PubMed] [Google Scholar]
- 18. Demetri GD, Garrett CR, Schoffski P, et al. Complete longitudinal analyses of the randomized, placebo-controlled, phase III trial of sunitinib in patients with gastrointestinal stromal tumor following imatinib failure. Clin Cancer Res 2012; 18: 3170–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013; 381: 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blay JY, Serrano C, Heinrich MC, et al. Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2020; 21: 923–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smith BD, Kaufman MD, Lu WP, et al. Ripretinib (DCC-2618) is a switch control kinase inhibitor of a broad spectrum of oncogenic and drug-resistant KIT and PDGFRA variants. Cancer Cell 2019; 35: 738–751.e739. [DOI] [PubMed] [Google Scholar]
- 22. Bai Y, Bandara G, Ching Chan E, et al. Targeting the KIT activating switch control pocket: a novel mechanism to inhibit neoplastic mast cell proliferation and mast cell activation. Leukemia 2013; 27: 278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Janku F, Abdul Razak AR, Chi P, et al. Switch control inhibition of KIT and PDGFRA in patients with advanced gastrointestinal stromal tumor: a phase 1 study of ripretinib. J Clin Oncol 2020; 38: JCO2000522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Serrano C, Heinrich MC, George S, et al. Efficacy and safety of ripretinib as ⩾4th-line therapy for patients with Gastrointestinal Stromal Tumor (GIST) following crossover from placebo: analysis from INVICTUS. In: European Society for Medical Oncology 22nd world conference on gastrointestinal cancer, Virtual Meeting, 1–4 July 2020. [Google Scholar]
- 25. George S, Heinrich MC, Zalcberg J, et al. Safety profile of ripretinib, including impact of alopecia and Palmar-Plantar Erythrodyesthesia Syndrome (PPES) on patient reported outcomes (PROs) in ⩾4th-line advanced Gastrointestinal Stromal Tumors (GISTs): analyses from INVICTUS. J Clin Oncol 2020; 38 (Suppl): Abstract 11539. [Google Scholar]
- 26. Zalcberg J, Heinrich M, George S, et al. Clinical benefit with ripretinib as ⩾4th line treatment in patients with advanced gastrointestinal stromal tumors (GIST): update from the phase 3 INVICTUS study. Presented at the European Society for Molecular Oncology Virtual Annual Meeting, 18 September 2020. [Google Scholar]
- 27. Gelderblom H, Heinrich M, George S, et al. Clinical benefit with ripretinib as ⩾fourth-line treatment in patients with advanced gastrointestinal stromal tumor: update from the phase 3 INVICTUS study. Presented at the Connective Tissue Oncology Society Virtual Annual Meeting, 18–20 November 2020. [Google Scholar]
- 28. Heinrich MC, George S, Zalcberg J, et al. Quality of Life (QoL) and self-reported function with ripretinib in ⩾4th-line therapy for patients with Gastrointestinal Stromal Tumors (GIST): analyses from INVICTUS. J Clin Oncol 2020; 38 (Suppl): Abstract 11535. [Google Scholar]
- 29. McHorney C, Cha E, Becker C. Thresholds for meaningful change for the EQ-to VAS and EORTC QLQ-C30 physical and role functioning scale in gastrointestinal-related cancers. Value Health 2020; 23(Suppl. 1): PG137. [Google Scholar]
- 30. Kang YK, Ryu MH, Yoo C, et al. Resumption of imatinib to control metastatic or unresectable gastrointestinal stromal tumours after failure of imatinib and sunitinib (RIGHT): a randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2013; 14: 1175–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nemunaitis J, Bauer S, Blay JY, et al. Intrigue: phase III study of ripretinib versus sunitinib in advanced gastrointestinal stromal tumor after imatinib. Future Oncol 2020; 16: 4251–4264. [DOI] [PubMed] [Google Scholar]
- 32. Deciphera Pharmaceuticals, LLC. Deciphera pharmaceuticals completes target enrollment in the INTRIGUE phase 3 clinical study of QINLOCK® (Ripretinib) in patients with second-line gastrointestinal stromal tumor. Press Release, https://investors.deciphera.com/news-releases/news-release-details/deciphera-pharmaceuticals-completes-target-enrollment-intrigue (2020, accessed 30 November 2020).