Abstract
A 61-year-old male patient was simultaneously diagnosed with lung adenocarcinoma and inflammatory myofibroblastic tumor (IMT). The lung adenocarcinoma and IMT harbored two distinct types of ALK translocation, LOC101927285-ALK, and TPM3-ALK, respectively. The ALK Ventana showed strong positivity on both lesions. The patient was therefore given an endobronchial cryotherapy and ALK inhibitor crizotinib. The tumors showed durable response however the left lung adenocarcinoma relapsed at 17th month post-crizotinib treatment. Tissue re-biopsy on the resistant tumor revealed an ALK exon 23 C1156Y missense mutation in addition to LOC101927285-ALK mutation. Further RNA-based sequence uncovered that the noncoding region rearrangement is the fusion mutation of EML4-ALK. The patient was therefore received alectinib, and the tumor exhibited partly response. Overall, it is very rare that two types of pulmonary tumors exist in one patient driven by two distinct ALK fusions, which emphasizes the necessity of gene sequencing in clinical decision-making and individualized therapy.
Keywords: ALK rearrangement, Crizotinib, Adenocarcinoma of lung, Inflammatory myofibroblastic tumor, Ventana, Alectinib
Introduction
Anaplastic lymphoma kinase (ALK) translocation occurs in only 3%–7% of all non-small cell lung cancer (NSCLC) patients [1]. Crizotinib was the first approved ALK inhibitor which was used as the first-line treatment for ALK-rearranged NSCLC patients. EML4-ALK rearrangement is the most frequent ALK fusion in NSCLC, however other rare fusion partners of ALK are also detected by next-generation sequencing (NGS). Moreover, previous studies have reported that approximately half of inflammatory myofibroblastic tumor (IMT) patients carry ALK rearrangements, who show sustained response to crizotinib [2]. In this letter, we share a case of simultaneously diagnosed lung adenocarcinoma and endobronchial IMT with two distinct types of ALK translocations.
Case Report
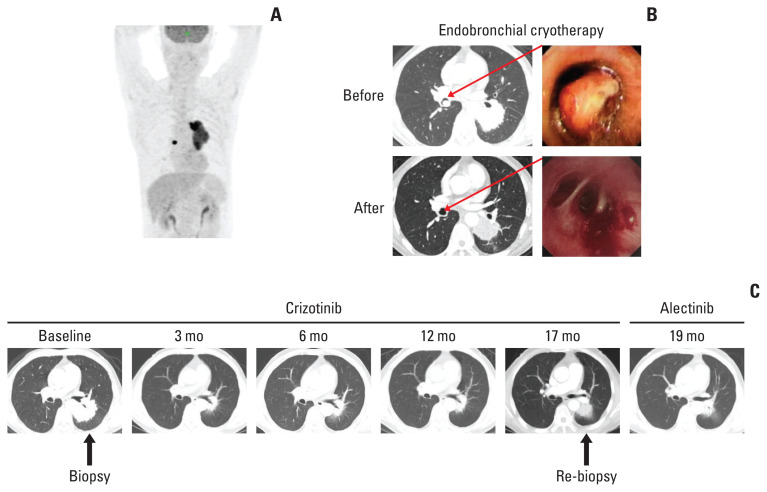
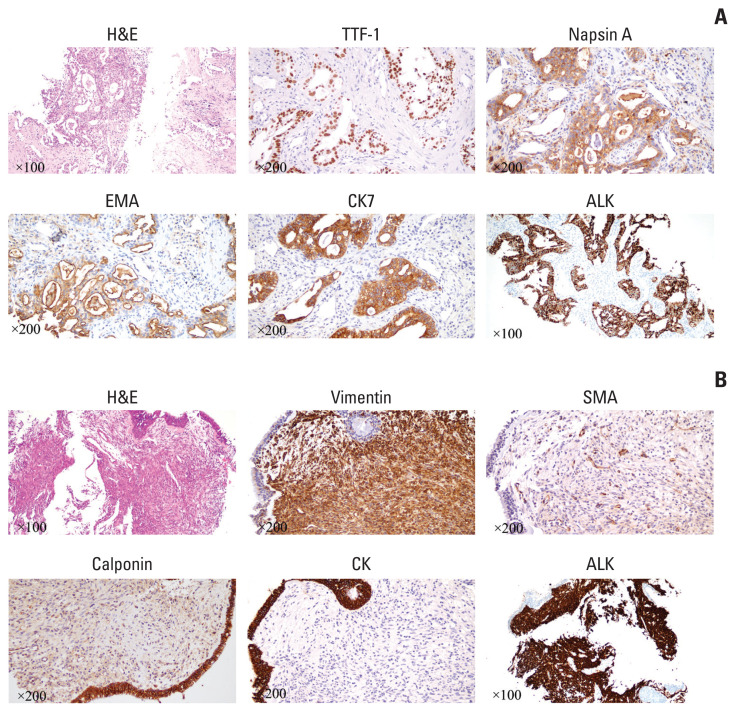
A 61-year-old male patient without a history of smoking was admitted to the hospital because of a persistent cough for one and a half years. Enhanced computed tomography (CT) indicated a subcircular lesion in the right intermediate bronchial trunk, which was confirmed by bronchoscopic examination (Fig. 1B), as well as a mass in the left lower lobe evading the posterior segment of the upper lobe that was approximately 6.4×4.6 cm in size (Fig. 1C). Positron emission tomography–CT examination exhibited significantly increased 18F-fluorodeoxyglucose uptake in both lesions with SUV values of 12.3 (left lower lobe) and 19.1 (right bronchial trunk), respectively (Fig. 1A). Except for slightly elevated cytokeratin-19 (4.43 ng/mL; range, 0 to 3.3 ng/mL), no other significantly physical, laboratory and radiological abnormalities were identified. The patient was given endobronchial cryotherapy to completely remove the lesion obstructing the intermediate bronchial trunk (Fig. 1B) and a CT-guided percutaneous biopsy for the left lung mass. Pathological examination showed that the left lung lesion was lung ade-nocarcinoma (Fig. 2A); the diagnosis of IMT was considered for the right endobronchial lesion because of the spindle cellular morphology and positive expression of vimentin, smooth muscle actin, and calponin as well as negative cytokeratin (Fig. 2B) [3].
Fig. 1.
Computed tomography (CT), positron emission tomography (PET)-CT, and bronchoscope findings. (A) PET-CT image prior the treatments. (B) Endobronchial inflammatory myofibroblastic tumor was shown by CT and bronchoscopic examination prior and post the endobronchial cryotherapy. (C) Enhanced chest CT images of left lung adenocarcinoma during the first-line crizotinib treatment and second-line alectinib treatment.
Fig. 2.
H&E and immunohistochemical staining of lung adenocarcinoma and inflammatory myofibroblastic tumor (IMT). (A) Positive expression of thyroid transcription factor 1 (TTF-1), napsin A, epithelial membrane antigen (EMA), cytokeratin (CK) 7, and anaplastic lymphoma kinase (ALK) Ventana in lung adenocarcinoma. (B) Positive expression of vimentin, smooth muscle actin (SMA), calponin, and ALK Ventana, as well as negative expression of CK for IMT.
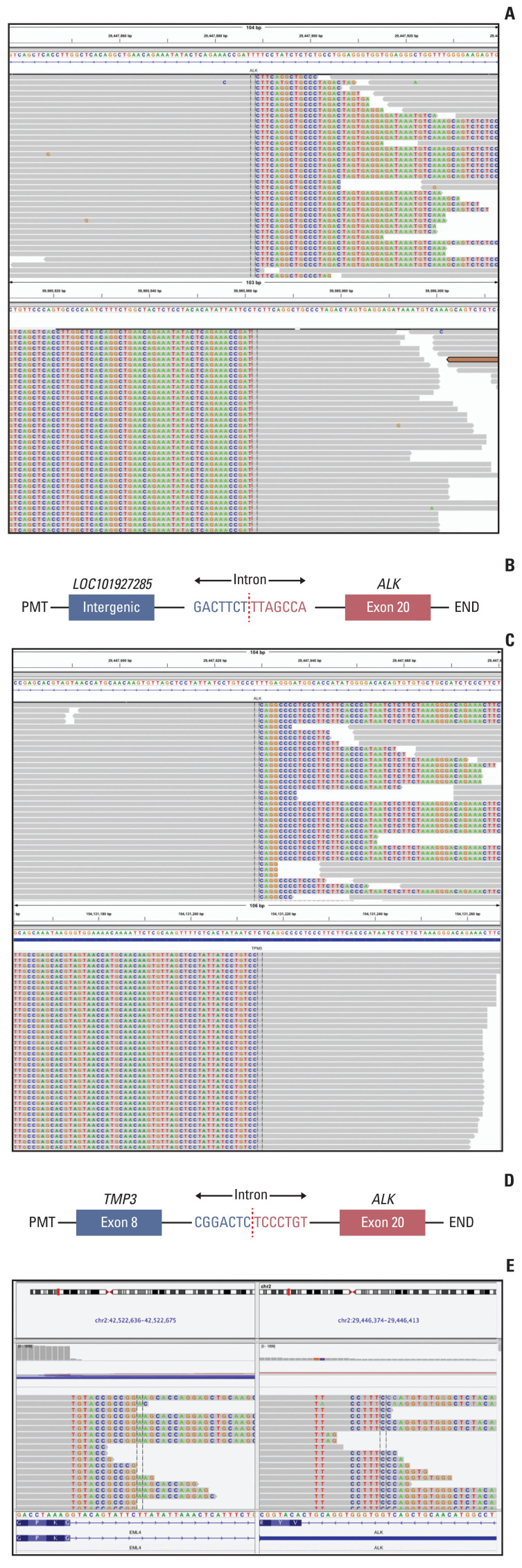
We further performed a genetic analysis for both lesions using capture-based targeted sequencing panels, consisting of 68 (for left lung adenocarcinoma) or 520 (for right IMT) cancer-related genes (Burning Rock Biotech, Guangzhou, China). The left lung adenocarcinoma exhibited an ALK translocation (LOC101927285-ALK, 22.92%) and CTNNB1 missense mutation (c.133T>C, 21.76%), and the right IMT showed a different ALK translocation (TPM3-ALK, 22.14%) and GNA11 missense mutation (c.844A>G, 23.85%) (Fig. 3A–D). TPM3-ALK rearrangement has been demonstrated to be the oncogenic driver and therapeutic target for IMT [4]. However, the LOC101927285-ALK translocation of the left lung adenocarcinoma, which was constituted by the end of the breakpoint located on the 19 intron of classical ALK and the other end of the breakpoint located on the noncoding region chr2:59985962, has not yet been reported. The ALK Ventana, which used anti-ALK (D5F3) rabbit monoclonal primary antibody (Roche, Basel, Switzerland), showed strong positivity on both lesions (Fig. 2), and the patient was therefore given the ALK inhibitor crizotinib. The left lung tumor showed significant shrinkage after 1 month of treatment and was continuously reduced three months later (Fig. 1C). And there was no sign of recurrence of IMT in the right bronchus.
Fig. 3.
Identification of anaplastic lymphoma kinase (ALK) fusion by next-generation sequencing. (A) Sequencing read of LOC101927285-ALK fusion is shown by the Integrative Genomics Viewer. (B) Schematic structure of the genomic DNA sequence shows fusion points for the LOC101927285-ALK fusion. (C) Sequencing read of TMP3-ALK fusion is shown by the Integrative Genomics Viewer. (D) Schematic structure of the genomic DNA sequence shows fusion points for the TMP3-ALK fusion. (E) The chimeric reads of RNA-based next generation sequencing showed EML4-ALK rearrangement.
After 17 months treatment of crizotinib, the chest CT scan shows the progression of the left lung adenocarcinoma. We then performed the re-biopsy and sequencing data showed an ALK exon 23 C1156Y missense mutation which is a known mechanism for resistance against crizotinib, in addition to LOC101927285-ALK mutation. To further explore the cause of rearrangement, we further performed a RNA-based NGS and the sequencing results showed that the true form of mutation is the classic EML4-ALK rearrangement. The second-generation of ALK tyrosine kinase inhibitor alectinib was then prescribed. CT scans after 2 months of alectinib treatment showed significantly radiologic response. The progression-free survival for alectinib treatment is 4 months now and the treatment is ongoing and well-tolerated.
Discussion
Crizotinib was approved by the U.S. Food and Drug Administration (FDA) for the treatment of patients with ALK-positive NSCLC in 2011 [5], which is also a potent and specific small-molecule inhibitor of ROS1 and c-MET tyrosine kinases. EML4-ALK is the most common gene fusion in NSCLC patients. Besides, a series of other ALK fusion partners have been discovered in NSCLC, including KIF5B, KLC1, and TPR, and most of these ALK fusions in NSCLC patients respond well to the ALK inhibitor crizotinib [6].
In our case, the DNA-based NGS suggested that LOC-101927-285-ALK rearrangement may form a LOC101927285-ALK fusion protein, which is probably inconsistent with the prediction of DNA breakpoint. But either way the positive Ventana staining and effectiveness of crizotinib indicates that this DNA rearrangement does lead to the activation of ALK fusion protein and drives the initiation of the tumor. The patient developed drug resistance at 17 months post-crizotinib treatment. Tissue re-biopsy and DNA-based NGS identified that the acquired C1156Y missense mutation conferred resistance to crizotinib. It is well-known that C1156Y has been reported as crizotinib-resistant mutation but is sensitive to alectinib, and we confirmed that resistant lung ade-nocarcinoma had a remarkable response to alectinib. More interestingly, DNA-based NGS on the resistant lung adenocarcinoma also detected rearrangement of ALK and noncoding region LOC101927285, however RNA-based sequencing confirmed that the true form of mutation is the classic EML4-ALK rearrangement. It is possible that the fragment of LOC101927285 was rearranged with intron 13 of EML4 to form a fusion protein of EML4-ALK.
Previous studies have shown that activation of ALK gene exists in some other tumors such as inflammatory myofibroblastic tumors, neuroblastomas, and diffuse large B-cell lymphomas, indicating that ALK-mediated signaling may contribute to the development or progression of these tumors [7]. As a soft tissue neoplasm which has a limited metastatic potential, IMT can occur at any age, and surgical resection is the first-line treatment for this tumor. Approximately half of the IMT patients have ALK rearrangements, and ALK inhibitors have showed good effect in the treatment of IMT patients with various ALK fusions. TPM3-ALK is the most frequent fusion gene described in IMT; and previous study suggests that IMT patient with TPM3-ALK rearrangement is responded to ceritinib after failure of crizotinib. This result indicates that ceritinib might be a proper choice after progression on crizotinib for patients with unresectable ALK-rearrangement IMT [4]. We also find GNA11 missense mutation exists in the IMT, which has never been reported. Previous study has shown that GNA11 missense mutation could reflect a more aggressive tumor phenotype in uveal melanoma [8], which may associate with a poor prognosis. In our case, the patient received endobronchial cryotherapy to remove the right endobronchial IMT, which was further controlled by crizotinib and alectinib. No sign of recurrence was found for the IMT in the right bronchus during the follow-up so far.
In a word, it is very rare that two types of pulmonary tumors exist in one patient driven by two distinct ALK fusions, which emphasizes the necessity of gene sequencing in clinical decision-making and individualized therapy.
Acknowledgments
We would thank Zhou Zhang and Bing Li (Burning Rock Dx, Guangzhou, China) for their technical support. The patient in this study provided written informed consent for specimen collection, genetic testing, and use of this information for research purposes.
The present study was funded by the National Natural Science Foundation of China (No. 81772464), and Tianjin Science and Technology Plan Project (19ZXDBSY00060).
Footnotes
Ethical Statement
This study was approved by Ethics Committee of Tianjin Medical University General Hospital and the patient provided his written informed consent for the publication of the case report. The patient agreed and submitted a written informed consent to allow publication of this report and the accompanying images. Institutional approval was not required to publish this manuscript.
Author Contributions
Conceived and designed the analysis: Liu W, Li S, Chen Q, Xu S.
Collected the data: Zhao S, Li Q, Sun L, Ren D, Song Z, Huang C.
Contributed data or analysis tools: Zhao S, Shi T, Chen Q, Sun L, Ren D, Song Z.
Performed the analysis: Zhao S, Li Q, Huang C, Xu S.
Wrote the paper: Zhao S, Shi T.
Conflicts of Interest
Conflicts of interest relevant to this article was not reported.
References
- 1.Shackelford RE, Vora M, Mayhall K, Cotelingam J. ALK-rearrangements and testing methods in non-small cell lung cancer: a review. Genes Cancer. 2014;5:1–14. doi: 10.18632/genesandcancer.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butrynski JE, D’Adamo DR, Hornick JL, Dal Cin P, Antonescu CR, Jhanwar SC, et al. Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N Engl J Med. 2010;363:1727–33. doi: 10.1056/NEJMoa1007056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montgomery EA, Shuster DD, Burkart AL, Esteban JM, Sgrignoli A, Elwood L, et al. Inflammatory myofibroblastic tumors of the urinary tract: a clinicopathologic study of 46 cases, including a malignant example inflammatory fibrosarcoma and a subset associated with high-grade urothelial carcinoma. Am J Surg Pathol. 2006;30:1502–12. doi: 10.1097/01.pas.0000213280.35413.1b. [DOI] [PubMed] [Google Scholar]
- 4.Mansfield AS, Murphy SJ, Harris FR, Robinson SI, Marks RS, Johnson SH, et al. Chromoplectic TPM3-ALK rearrangement in a patient with inflammatory myofibroblastic tumor who responded to ceritinib after progression on crizotinib. Ann Oncol. 2016;27:2111–7. doi: 10.1093/annonc/mdw405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kazandjian D, Blumenthal GM, Chen HY, He K, Patel M, Justice R, et al. FDA approval summary: crizotinib for the treatment of metastatic non-small cell lung cancer with anaplastic lymphoma kinase rearrangements. Oncologist. 2014;19:e5–11. doi: 10.1634/theoncologist.2014-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du X, Shao Y, Qin HF, Tai YH, Gao HJ. ALK-rearrangement in non-small-cell lung cancer (NSCLC) Thorac Cancer. 2018;9:423–30. doi: 10.1111/1759-7714.12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camidge DR, Bang YJ, Kwak EL, Iafrate AJ, Varella-Garcia M, Fox SB, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;13:1011–9. doi: 10.1016/S1470-2045(12)70344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dono M, Angelini G, Cecconi M, Amaro A, Esposito AI, Mirisola V, et al. Mutation frequencies of GNAQ, GNA11, BAP1, SF3B1, EIF1AX and TERT in uveal melanoma: detection of an activating mutation in the TERT gene promoter in a single case of uveal melanoma. Br J Cancer. 2014;110:1058–65. doi: 10.1038/bjc.2013.804. [DOI] [PMC free article] [PubMed] [Google Scholar]