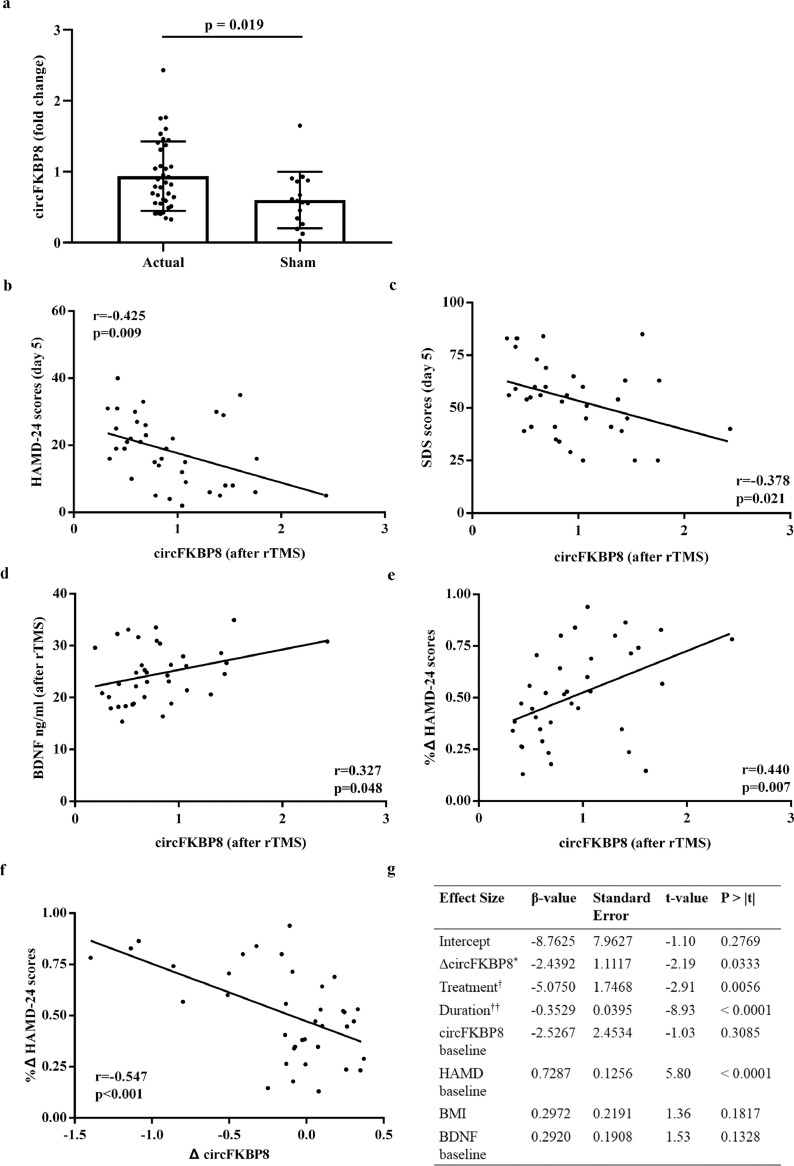
Fig. 3.
Potential clinical value of circFKBP8 in the longitudinal study. (a) Expression levels of circFKBP8 in MDD patients between actual rTMS treatment group (n = 37) and sham treatment group (n = 16). The relative fold change of actual and sham groups were calculated using healthy controls as the reference, respectively. Each sample was tested in triplicate. Independent-samples t-test was used for data analysis and all data represents means ± standard deviation. (b) – (d) Correlation between circFKBP8 expression and HAMD-24 scores, SDS scores and serum BDNF levels in MDD patients at the conclusion of actual rTMS treatment (n = 37). (e) Correlation between rates of change of HAMD-24 scores and circFKBP8 expression in MDD patients at the conclusion of actual rTMS treatment (n = 37). (f) Correlation between rates of change of HAMD-24 scores and change in circFKBP8 expression in 37 MDD patients with rTMS treatment. (g) Model of change in value of circFKBP8 expression for prediction of HAMD-24 scores following rTMS treatment in 53 MDD patients. * Three sub-groups of MDD patients were obtained based on tertiles of change in circFKBP8 expression; † Influence of rTMS and sham treatments were considered in the model; †† Duration from the end of rTMS treatment to the end of the 4-week follow-up period. MDD, major depressive disorder; HC, healthy control; rTMS, repetitive transcranial magnetic stimulation; HAMD-24, 24-item Hamilton Depression Rating Scale; SDS, Self-Rating Depression Scale; BDNF, brain-derived neurotrophic factor; BMI, body mass index.