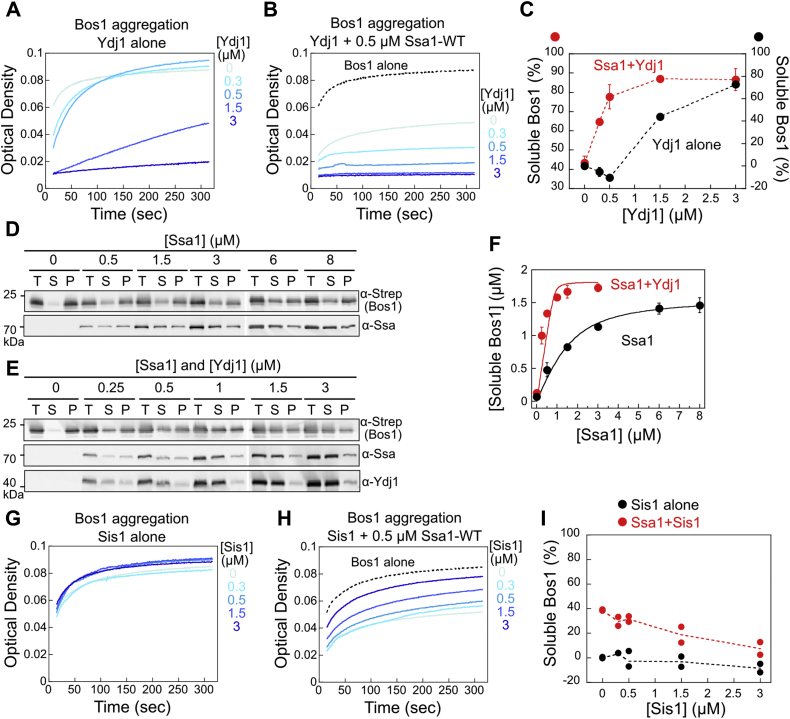
Figure 2.
Ydj1 cooperates with Ssa1 to suppress Bos1 aggregation in vitro, but Sis1 does not.A and B, time courses of Bos1 aggregation in the presence of indicated concentrations of Ydj1 without (A) and with (B) 0.5 μM Ssa1 present. C, quantification of the data in (A), (B), and their replicates. Note the different y-axis scales for the data with and without Ssa1. D and E, representative Western blot analyses of sedimentation experiments to measure the solubility of 3 μM Bos1 in the presence of Ssa1 (D) or equimolar mixtures of Ssa1 and Ydj1 (E) at the indicated concentrations. “T”, “S”, and “P” represent total input, soluble, and pellet, respectively. F, quantification of the concentration of soluble Bos1 from the data in (D), (E), and their replicates. The data were fit to Equation 2, which gave KSoluble values of 0.73 ± 0.012 μM with Ssa1 and 0.014 ± 0.003 μM with both Ssa1 and Ydj1 present. G and H, time courses of Bos1 aggregation in the presence of indicated concentrations of Sis1 without (G) and with (H) 0.5 μM Ssa1 present. I, quantification of the data in (G), (H), and their replicates. All values in (C) and (F) are reported as mean ± SD, with n = 3. Error bars are shown but may not be visible in some cases. The values from two independent experiments are shown in (I) as black (Ssa1 alone) and red (Ssa1+Sis1) circles.