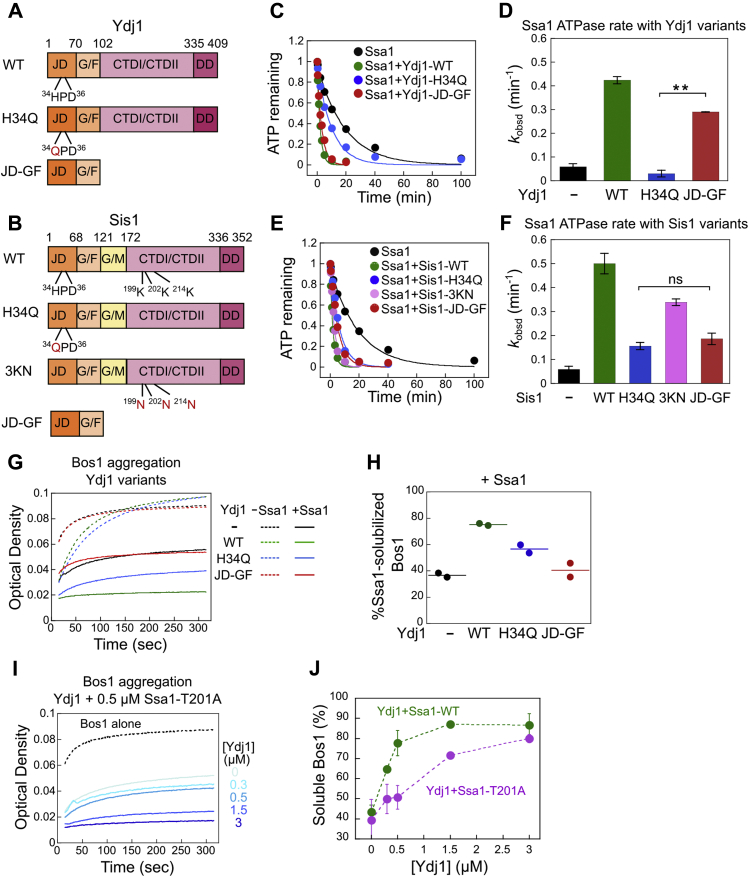
Figure 4.
Ydj1 assists Ssa1 in TA capture in a soluble form using both its CTDs and J-domain.A and B, schematic of the domain organization of wildtype and mutant Ydj1 (A) and Sis1 (B) tested in this work. C–F, representative time courses for single-turnover ATP hydrolysis reactions were shown for reactions with 3 μM Ssa1 in the absence and presence of 3 μM wildtype and mutant Ydj1 (C) or Sis1 (E). ATPase reactions were measured and analyzed as described under Experimental procedures. Lines are fits of the data to Equation 3, and the obtained ATPase rate constants are summarized in parts (D) and (F). G, time courses of Bos1 aggregation in the presence of 0.5 μM of the indicated Ydj1 variants with (solid lines) and without (dotted lines) 0.5 μM Ssa1. H, the difference in optical density between the (−Ssa1) and (+Ssa1) reactions in (G) and their replicates are quantified. The lines represent the mean from two independent experiments. I, time courses of Bos1 aggregation in the presence of 0.5 μM Ssa1(T201A) and indicated concentrations of Ydj1. J, quantification of the data in (I) and their replicates (purple). The data for 0.5 μM Ssa1-WT and Ydj1 (green) were from Figure 2C and shown for comparison. All values in (D), (F), and (J) are reported as mean ± SD, with n ≥ 3. “∗∗” denotes p < 0.001 from Student's t test. Error bars are shown but may not be visible in some cases. CTDI/CTDII, C-terminal domains I and II; DD, dimerization domain; G/F, Glycine and Phenylalanine-rich linker; G/M, Glycine and Methionine-rich region; JD, J-domain; TA, tail-anchored protein.