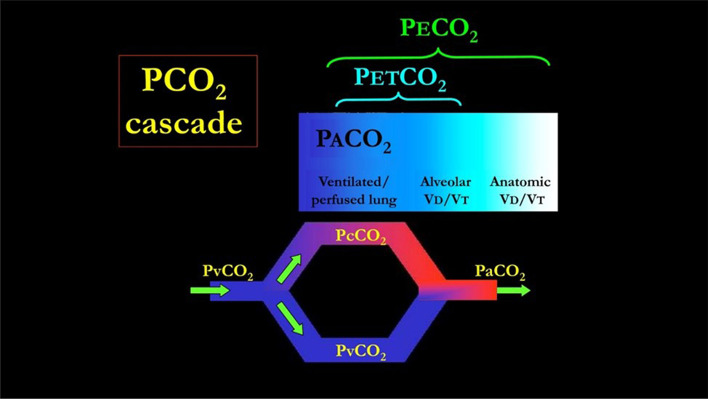
Fig. 4.
The PvCO2 decreases to PcCO2 (the capillary PCO2 pressure) after releasing CO2 in the alveolar space. The partial pressure of CO2 in the alveoli (PACO2) which are perfused is equal to PcCO2. This pressure represents the “ideal” alveolar gas, as it derives from pulmonary units which are both perfused and ventilated. This model ignores other factors possibly affecting the CO2 dynamics, such as the CO2 production from the inflammatory cells residing in the inflamed lung. Actually, this ideal PACO2 may be “diluted” by the gas coming from unperfused but ventilated alveoli (alveolar dead space). The PACO2 then becomes PETCO2. In turn, the PETCO2 may be diluted into the gas ventilating the airways and the apparatus (anatomical dead space) becoming PETCO2. It must be realized that the number of molecules of CO2 involved are exactly the same, but their concentration (and partial pressure) depends on the progressive dilution with alveolar gases and “anatomical” gases. In normal individual, PACO2 and PETCO2 differ only of 1–2 mmHg, being the alveolar dead space negligible. The PvCO2 may also reach the arterial circulation without any modification in presence of venous admixture. In this case, PaCO2 is the result of mixing between PcCO2 and PvCO2. Greater is the venous admixture, greater is the difference between PaCO2 (higher) and PcCO2. In the perfect gas exchanger, PETCO2 equals the PACO2 (no alveolar dead space) and PACO2 equals PaCO2 (no venous admixture). Therefore PETCO2/PaCO2 equals 1