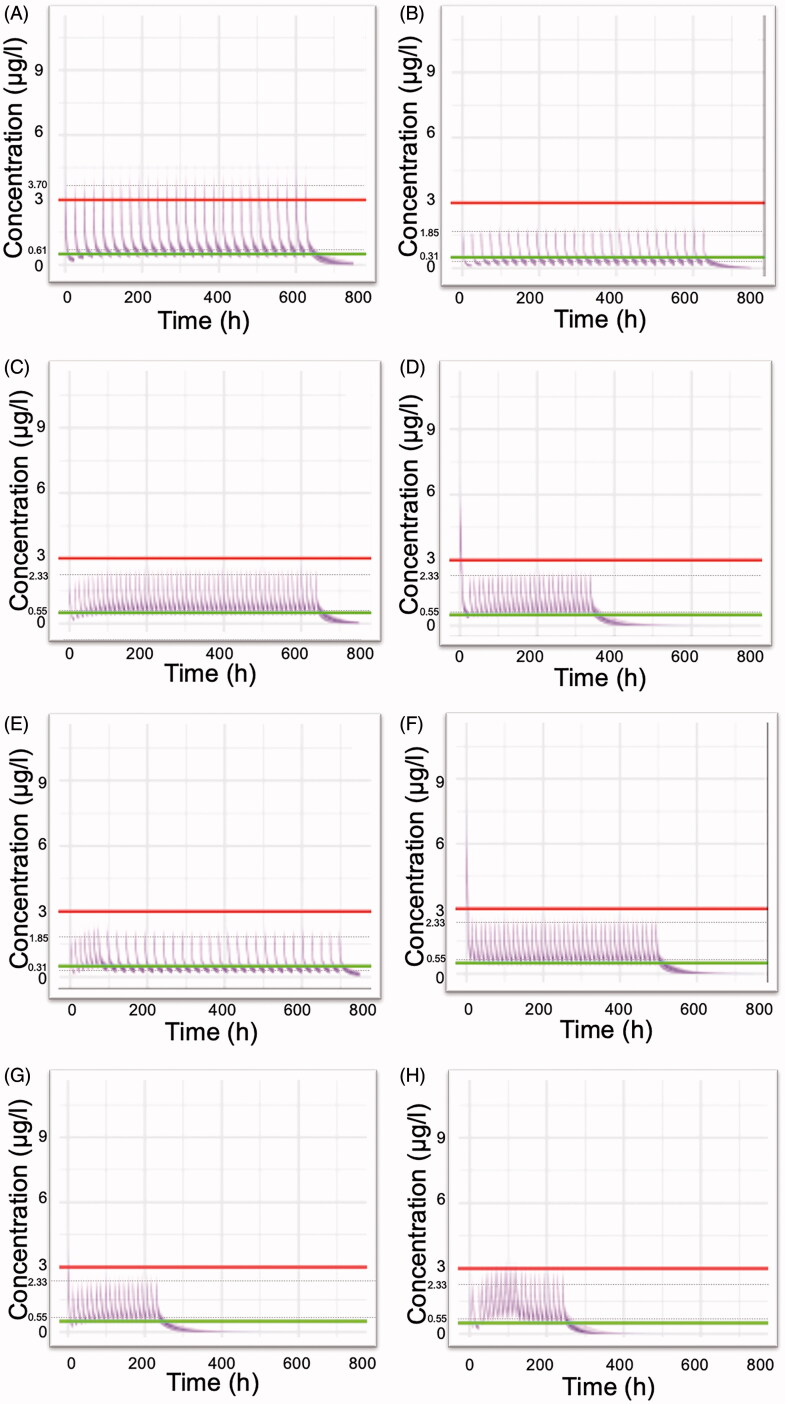
Figure 2.
Simulated plasma colchicine concentrations (μg/l) versus time (h) according to dosing schemes (Table 4) currently used in clinical practice or in clinical trials. (A) 1 mg once daily, (B) 0.5 mg once daily, (C) 0.5 mg twice daily, (D) Loading dose of 1.5 mg, followed by 0.5 mg after 2 h and then 0.5 twice daily for 14 days, (E) COLCORONA trial: 0.5 mg twice daily for the first 3 days and then 0.5 once daily for 27 days, (F) GRECCO-19 trial: Loading dose 1.5 mg, followed by 0.5 mg after 1 h and then 0.5 twice daily. (G) RECOVERY trial: Loading dose of 1 mg, followed by 0.5 mg 12 h later, then maintenance dose 0.5 mg twice daily for a further of nine days (10 days in total) or until hospital discharge, (H) 0.5 mg thrice daily for 5 days, then 0.5 mg twice daily for 5 days. Upper line: 3 μg/l, plasma concentration that elicits toxic effects. Lower line: 0.5 μg/l, minimum effective plasma concentration. The inter-individual variability values, considered for all pharmacokinetic parameters, were those reported in the study of Thomas et al. (1989). Dashed lines indicate the average peak and trough concentration values at steady state.