Fig. 2.
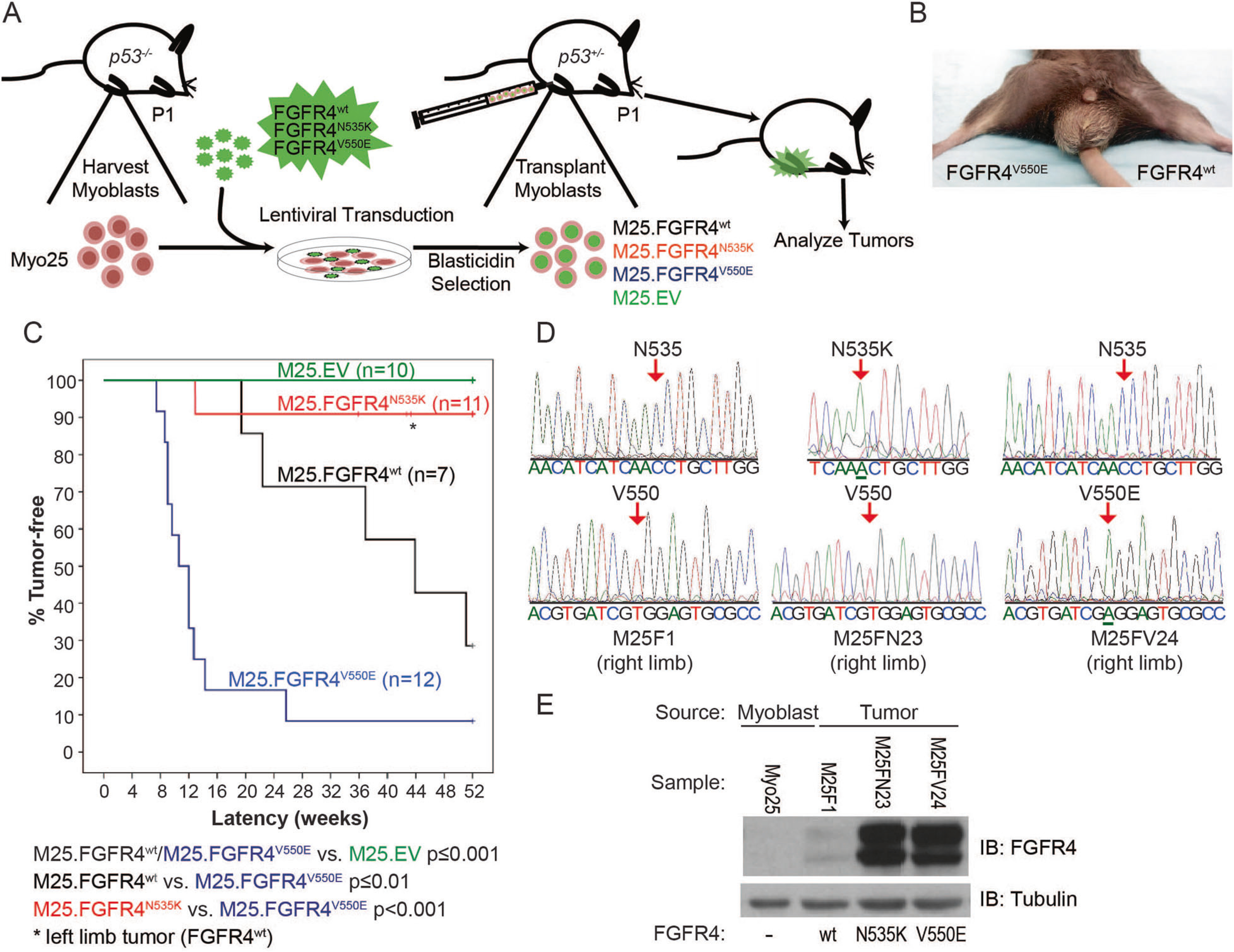
FGFR4V550E p53−/− myoblasts engrafted into murine skeletal muscle generate a high incidence of tumors. a Murine model schema depicts p53−/− myoblast harvest, transduction, injection into p53+/− host hind limbs, and tumor analysis. b A murine tumor following M25. FGFR4V550E injection into the right hind limb. c Kaplan–Meier curves depicting tumor-free survival illustrate statistically significant differences among tumor models expressing different FGFR4 constructs. M25.EV did not generate tumors. M25.FGFR4V550E- and M25. FGFR4wt-injected mice formed tumors with statistically different penetrance and latencies compared to each other and to the M25.EV control cohort. Two mice injected with M25.FGFR4N535K developed tumors, one encoded FGFR4N535K while the second tumor was shown to encode FGFR4wt (asterisk). d Amplicons from tumor tissue were sequenced and shown to encode the correct FGFR4 transgenes. M25F1 represents tumor tissue from the M25.FGFR4wt cohort, M25FN23 from M25.FGFR4N535K, and M25FV24 from M25. FGFR4V550E. e Protein expression was confirmed in tumor lysates using immunoblot and human FGFR4-specific antibodies. Parental myoblasts, Myo25, did not express human FGFR4 proteins
