Fig. 3.
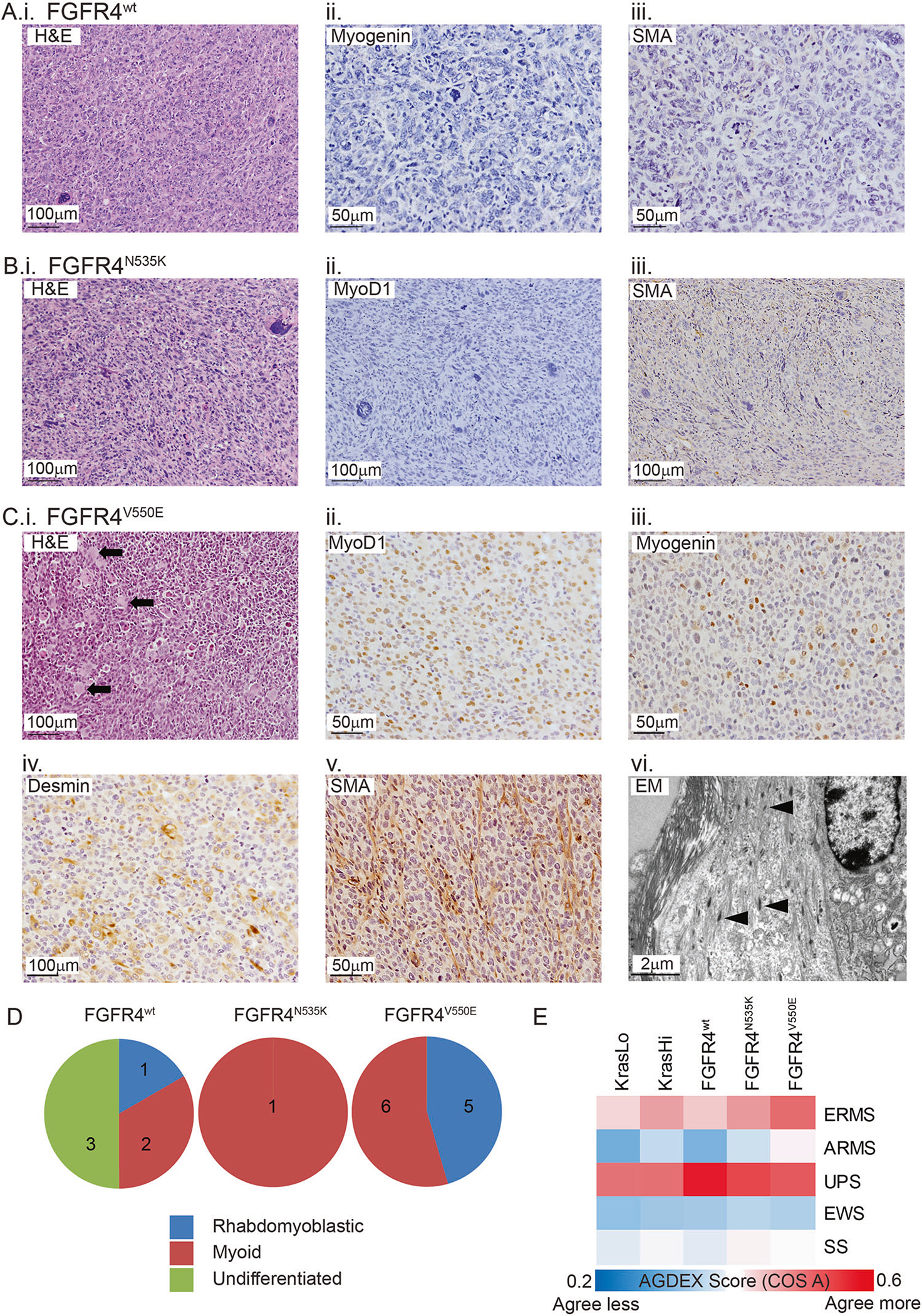
Histopathology of FGFR4-expressing sarcomas is consistent with rhabdomyoblastic differentiation. a Tumors were subjected to morphological analysis using H&E-stained sections and classified following immunohistochemical labeling with a panel of diagnostic markers. Half of the tumors generated following M25. FGFR4wt injection were undifferentiated (i) and did not commonly express the muscle-specific proteins Myogenin (ii) and SMA (iii). b The only M25. FGFR4N535K tumor was classified as a high-grade sarcoma (i) with myoid differentiation based on the absence of Myod1 protein (ii) and presence of SMA (iii). c All tumors generated from M25. FGFR4V550E-injected host mice were high-grade sarcomas (i) with muscle differentiation (myoid/rhabdomyoblastic) based on the presence of rhabdomyoblasts (black arrows) and expression of Myod1 (ii), Myogenin (iii), Desmin (iv), or SMA (v). Transmission electron microscopy showed tumor cells containing immature muscle fibers (vi, black arrowheads). d Pie charts illustrate classification of the high-grade sarcomas among the three injected cohorts. e Heat map reporting the transcriptomic agreement (AGDEX score) in comparisons between KRAS- or FGFR4-driven mouse sarcomas and five subtypes of human sarcoma. EWS represents Ewing sarcoma and SS represents synovial sarcoma
