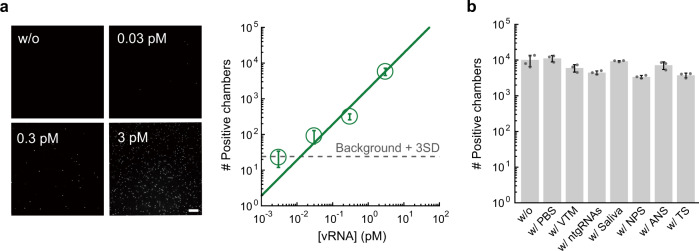
Fig. 4. Practicability of SATORI for clinical applications.
a Digital detection of RNA extracted from SARS-CoV-2 virus (vRNA). The representative fluorescence images and the number of positive chambers, obtained with the crRNA-CoV-N1 at different concentrations of vRNA, are shown. b Effects of contaminants on SATORI. SATORI assays were performed with the crRNA1 and the tgRNA1 in the presence of 10% PBS, 70% virus transport medium (VTM), 3 ng/μL nontarget RNAs (ntgRNAs), 10% saliva, nasopharyngeal swab (NPS), anterior nasal swab (ANS), or throat swab (TS).