Abstract
Heliotropium thermophilum can survive at a soil temperature of 65 °C in natural and laboratory conditions, but the mechanism of survival at high soil temperatures is not completely known. The objective of this study was to determine whether changes in abscisic acid (ABA), osmolytes and heat shock factors (HSFs) levels have an effective role in the development of thermotolerance in H. thermophilum at high temperatures. Soil temperature at which the thermophilic plant could live was gradually increased in laboratory conditions and the effects of four different temperatures (20 ± 5 °C: low, 40 ± 5 °C: mild, 60 ± 5 °C: medium, 80 ± 5 °C: extreme heat) were observed for 15 days. The results showed that the content of thiobarbituric acid reactive substances (TBARS) did not significantly change in extreme heat, whereas the leaf water potential and stomatal conductivity decreased. ABA biosynthesis, accumulation of osmolyte compounds including proline and total soluble sugars, and the expression levels of heat shock transcription factor A4A (HSFA4A), heat shock transcription factor A3 (HSFA3), and heat shock factor (HSF4) genes significantly increased with increase of soil temperature from 20 ± 5 °C to 80 ± 5 °C. In conclusion, we observed that H. thermophilum is an extreme thermophile. This plant can adjust osmotic activity to effectively take water through the osmolytes accumulation, reducing water loss by ABA-mediated stomatal closing and survive at high soil temperatures by stimulating the increased transcription level of HSFs.
Keywords: Heliotropium thermophilum, Thermophile, Abscisic acid, Osmolyte, Heat shock factors
Introduction
The increase in overall temperature of the earth caused by global warming negatively affect plant growth and productivity (Bita and Gerats 2013). Plants show physical changes in their habitats under high temperature stress resulting in reduced performance of plant cell functions including enzyme activity, membrane fluidity, formation of protein complexes, chlorophyll synthesis, photosynthesis, respiration, and redox status (Li et al. 2013). The response of plants to high temperatures depends on the degree and duration of heat stress. Plants can adapt in various ways to deal with high temperatures including avoidance and tolerance. Avoidance mechanisms include changes in leaf orientation, transpirational cooling, leaf rolling, early maturation, and membrane lipid composition (Fitter and Hay 2002). Tolerance mechanisms consist of cellular mechanisms involving ion carriers, proteins, osmoprotectants, antioxidants, signal cascades, and transcriptional control (Hasanuzzaman et al. 2013).
One of the most common responses in many plant species exposed to different abiotic stresses is the accumulation of stress hormone abscisic acid (ABA), which regulates various physiological processes (Ciura and Kruk 2018), which is also responsible for accumulation of protective proteins (late embryogenesis abundant proteins, dehydrins, heat shock proteins (HSPs)), osmoprotectants, stimulation of antioxidant capacity, and protection of turgor (Islam et al. 2018). Plants respond to adverse conditions in developmental, physiological, and biochemical ways, which require modulation of expression of stress-responsive genes regulated by a network of transcription factors (TFs), including HSFs, which play important roles in response towards different abiotic stresses in plants by regulating the expression of stress-responsive genes such as heat shock proteins (HSPs) (Meng et al. 2016). In our unpublished RNASeq data for Heliotropium thermophilum, the expression levels of HEAT SHOCK TRANSCRIPTION FACTOR A4A (HSFA4A), HEAT SHOCK TRANSCRIPTION FACTOR A3 (HSFA3), and HEAT SHOCK FACTOR 4 (HSF4) were elevated in high soil temperature conditions (65 °C) compared to the low soil temperature conditions (25 °C). ABA-induced accumulation of HSPs and associated heat tolerance in some plants species has been reported (Campbell et al. 2001; Li et al. 2014). However, it is yet to be ascertained whether heat tolerance in H. thermophilum in extreme heat (80 ± 5 °C) is due to regulation of HSFA4A, HSFA, and HSF4 genes. It is also known that increased synthesis of compatible osmolytes (proline, glycine betaine soluble sugars, polyols etc.) may adjust the cellular osmotic potential, allowing the recapture of soil water and maintenance of water potential in plant tissues under high temperature (Iba 2002). Since various abiotic stress factors involving heat stress result in different effects on osmolytes accumulation, a better understanding of the relationship between abiotic stress and osmolytes accumulation in plants could be obtained under controlled conditions by the application of these stress factors.
Plants with various tolerance mechanisms are more successful in surviving stress conditions. Indeed, thermophilic plants can grow more easily at high temperatures, and protect themselves against sudden temperature changes. Although, the number of plants that can grow above 45 °C is limited, there are several known examples in the literature. Thermal Agrostis scabra is a predominant grass species in geothermal areas which can grow at soil temperatures up to 45–50 °C (Tercek et al. 2003). In addition, thermophilic Cyclosorus interruptus and Kunzea robusta were found to grow in soil where temperatures reached 68 °C, while Campylopus pyriformis grows in soil at an average temperature of 72 °C (Lange 2014; Smale and Fitzgerald 2015). Heliotropium thermophilum, a thermophilic flowering plant endemic to Turkey, can grow in natural geothermal areas where soil temperatures range between 55 °C and 65 °C (Tan et al. 2008). Our research group reported that this plant was able to adapt at high temperature (60 °C) under laboratory conditions as well, and it does not seem to be stressed at 60 °C in natural or laboratory conditions (Oztürk et al. 2020). In the present study, H. thermophilum was exposed to 80 ± 5 °C soil temperature for the first time under laboratory conditions. Heliotropium thermophilum is able to survive extreme temperatures which marks it out as an important plant species to study the mechanisms responsible for survival under high soil temperatures. To the best of our knowledge there is no study reported about this mechanism in the literature. The goal was to determine whether changes in ABA, osmolytes and HSFs levels have an effective role in the development of thermotolerance in H. thermophilum. For this purpose, it was hypothesized that (1) H. thermophilum may be extremely thermophilic plant which could survive at extreme soil temperature (80 ± 5 °C), (2) the extreme thermophile, H. thermophilum could evoke osmotic adjustment, (3) H. thermophilum could highly stimulate the increased transcription level of HSFs and ABA biosynthesis under extreme soil temperatures.
Materials and methods
Plant material, growth conditions, and heat treatments
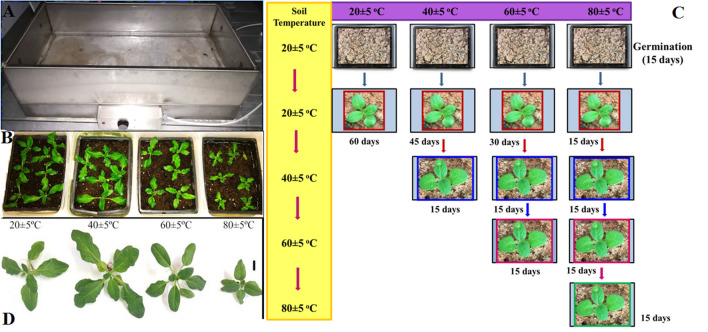
Heliotropium thermophilum, is an endemic plant, whose seeds were collected from the Buharkent geothermal area in Aydın Province in Turkey, where the soil temperature is 55–65 °C. The seeds were exposed to cold shock at 4 °C for 2 days on moistened filter paper in a petri dish before germination. The seeds were sown in containers containing soil from the natural environment. The plants were grown in a heating unit under laboratory conditions (23 ± 2 °C, 50% humidity, light intensity 400 μmol m−2 s−1, 16 h light/8 h dark period) in a walk-in growth chamber (DigiTech PG42). The temperature of the soil was kept at 20 ± 5 °C for maximum germination of seeds in habitat (Tan et al. 2008) and all plants were grown at this temperature for 15 days for acclimatization. Then the plants were divided into four temperature groups: 20 ± 5 °C (low), 40 ± 5 °C (mild), 60 ± 5 °C (medium), and 80 ± 5 °C (extreme). Temperature was increased stepwise, 20 °C every 15 days to 40, 60, and finally 80 °C. The temperature of the soil was periodically monitored by a digital thermometer at a depth of 5 cm. After the soil temperature reached to 80 ± 5 °C, the plants were kept in a growth chamber for 15 days for each temperature. The establishment of experimental groups and scheme of applications has been shown in Fig. 1c. All plants were regularly watered every day and harvested after 75 days. The leaves of the plants were used for following analysis.
Fig. 1.
The heating unit (a), Plants grown in the heating unit (b), Establishment of experimental groups and scheme of applications. Plant photographs were used to schematize the experimental setup (c), Heliotropium thermophilum growing in soil at 20 ± 5 °C, 40 ± 5 °C, 60 ± 5 °C and 80 ± 5 °C (d)
Determination of thiobarbituric acid reactive substances (TBARS) content
The level of lipid peroxidation was measured in terms of thiobarbituric acid-reactive substances (TBARS) contents as described by Heath and Packer (1968). Leaf samples (0.1 g) were homogenized in 10 mL of 0.1% trichloroacetic acid (TCA) by a homogenizer (TissueLyser LT, Qiagen). The homogenate was centrifuged at 15,000 xg for 5 min. 0.5 mL of thiobarbituric acid prepared in 4 mL of 20% TCA was added to 1 mL of the supernatant. The mixture was heated at 95 °C for 30 min and then quickly cooled in an ice bath. After 10 min centrifugation at 10,000 xg, the absorbance of the supernatant was recorded at 532 nm. The value for non-specific absorption at 600 nm was substracted. The TBARS content was calculated using its absorption coefficient of 155 mmol−1 cm−1.
Determination of leaf water potential
Leaf water potential was measured using the water potential system (Psypro P2-132 Water Potential System) according to Savage and Cass (1984).
Determination of ABA content
100 mg of fresh leaf samples were lyophilized for 3 h. Lyophilized samples were extracted in MilliQ water (Water/tissue ratio 50:1, v/w) at 4 °C for 16 h. Quantitative ABA analyses were performed with Phytodetek ABA ELISA kit. ( ±) cis–trans ABA (Sigma, St. Louis) was used as a standard.
Determination of stomatal conductivity
Stomatal conductivity values (gs) were taken by dynamic diffusion porometer (AP4 Delta T, UK). The calibration of the instrument was performed with a standard calibration plate according to the manufacturer's instructions (Cohen et al. 1987).
Determination of osmolyte contents
For proline content, dried samples (0.2 g) were taken and filtered after homogenization with 10 mL of 3% sulfosalicylic acid. The filtrate was centrifuged at 4000 xg for 5 min.1 mL of supernatant was taken and 1 mL of acetic acid and 1 mL of ninhydrin were added. The samples were then placed in tubes at 100 °C for 1 h in a water bath. 3 mL of toluene was added to the cooled samples and vortexed. Then absorbance was read on a spectrophotometer at 520 nm (Bates et al. 1973).
For glycine betaine content, the extract prepared with finely ground dry plant material (0.1 g) is mechanically shaken with 20 mL of deionized water for 48 h at 25 °C. The samples filtrated was diluted 1:1 with 2 N sulfuric acid. Then, potassium iodide-iodine reagent (0.2 mL) was added and centrifuged at 10,000 xg for 15 min. The supernatant (1 mL) was dissolved in 9 mL 1,2-dichloro ethane and the absorbance was measured at 365 nm (Greive and Grattan 1983).
For total soluble sugar content, after the dry leaf sample (0.2 g) was homogenized with 5 mL of 70% ethanol, the homogenate was boiled at 80 °C for 3 min then centrifuged at 10,000 xg for 5 min. 900 µL of distilled water was added to 100 µL of the supernatant. This mixture was added to 1 mL of 5% phenol and 5 mL of 96% sulfuric acid and their absorbance were measured at 490 nm (Dubois et al. 1956).
Gene expression analysis
Total RNA isolation was performed using Total RNA Isolation Kit (Quiagen RNeasy Plant Mini Kit (Cat. No: 74904). For the cDNA synthesis, Applied Capacity cDNA Reverse Transcription Kit (cat. no: 4368814, Applied Biosystems) was used. The resulting cDNAs were used in Real Time PCR assays to determine transcript level. For analysis, 5 × HOT FIREPol Eva Green qPCR Supermix (08–36-00,008, Solis Biodyne) and CFX Connect Real Time PCR System (BioRad) were used. RT-PCR process steps were modified by Solis BioDyne instructions: 95 °C for 12 min, 45 cycles of 95 °C for 15 s, 60 °C for 30 s, and 72 °C for 30 s, and a melt curve was held in 0.5 °C increments from 60 °C to 95 °C. Actin 7 gene was used as the reference gene for H. thermophilum. Data were normalized according to reference gene expression level and expressed as relative gene expression. Primers belong to HSFA4A, HSF4 and HSFA3 genes are listed in Table 1, which were designed using NCBI (The National Center for Biotechnology Information) database.
Table.1.
Sequences of primers used in qRT PCR
| Primers | Sequences of primers |
|---|---|
| ACT 7 | ACT 7-F: TACGAGCAGGAGCTTGACAC |
| ACT 7-R: CCGATCATGGAAGGCTGGAA | |
| HSFA4A: HEAT SHOCK TRANSCRIPTION FACTOR A4A | HSFA4A-F: TTCACCGACGCAAGCCAATA |
| HSFA4A-R: TTATGCCTCTCAAGCTCCGC | |
| HSF4: HEAT SHOCK FACTOR 4 | HSF4-F: GCTTCGTTCGCCAGCTTAAC |
| HSF4-R: TTCGGCGGCCTATTTTCGTA | |
| HSFA3: HEAT SHOCK TRANSCRIPTION FACTOR A3 | HSFA3-F: CCTCCTAATAGTACGCCGCC |
| HSFA3-R: CAAGGGGAGAGGCCATTGTT |
Statistical analysis
Statistical analyses were carried out with one-way ANOVA variance analysis tests (Duncan Multiple Comparison Test) using Statistical Package for Social Sciences (SPSS). P < 0.05 was considered statistically significant.
For qRT-PCR analysis, the relative gene expression level was analyzed using Bio-Rad CFX Manager 3.1. Expression levels were tested with SPSS software. Analysis of variance mean values were performed with one-way ANOVA (P ≤ 0.05).
Results
TBARS content
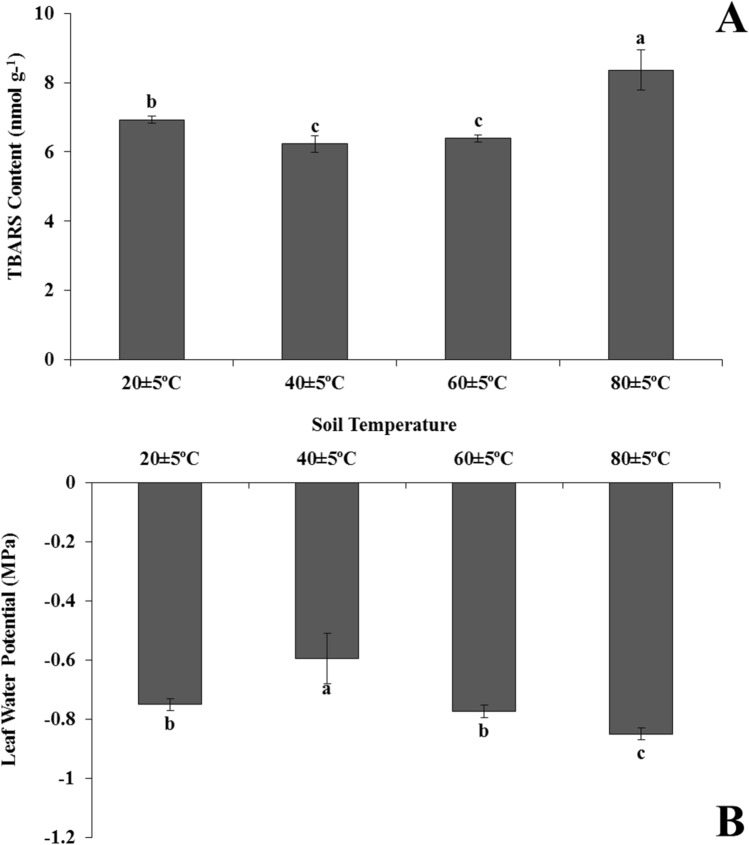
TBARS content in the plants grown at 80 ± 5 °C was 1.2, 1.3 and 1.3-times higher than 20 ± 5 °C, 40 ± 5 °C and 60 ± 5 °C temperature groups, respectively. On the other hand, membrane damage in plants growing in soil at 40 ± 5 °C and 60 ± 5 °C was lower compared to plants at 20 ± 5 °C and 80 ± 5 °C (Fig. 2a).
Fig. 2.
Changes of TBARS content (a), leaf water potential (b) in the leaves of Heliotropium thermophilum growing in soil at 20 ± 5 °C, 40 ± 5 °C, 60 ± 5 °C and 80 ± 5 °C. Vertical bars represent standard deviations of the means of three replicates. Different letters denote significant differences among all treatments at P < 0.05
Leaf water potential
Leaf water potential of the plants at 80 ± 5 °C was 1.1, 1.4 and 1.1-times lower than 20 ± 5 °C, 40 ± 5 °C and 60 ± 5 °C temperature groups, respectively. The highest leaf water potential was measured in plants subjected to 40 ± 5 °C compared to the 20 ± 5 °C, 60 ± 5 °C and 80 ± 5 °C temperature groups. Moreover, there was no statistically significant difference between the plants belonging to 20 ± 5 °C and 60 ± 5 °C groups (Fig. 2b).
ABA content
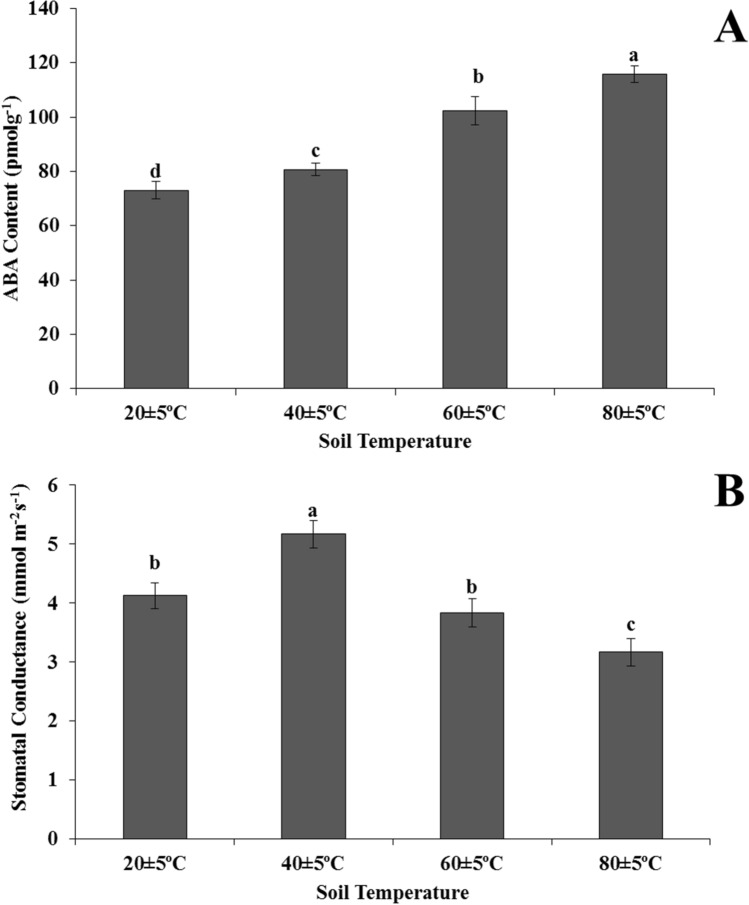
ABA content increased with rising soil temperatures from 20 ± 5 °C to 80 ± 5 °C. The highest increase in the ABA content was observed at the 80 ± 5 °C group. As compared to 20 ± 5 °C, 40 ± 5 °C and 60 ± 5 °C, there were 1.6-times, 1.4-times and 1.1-times increases in ABA content in plants growing in soil at 80 ± 5 °C, respectively (Fig. 3a).
Fig. 3.
Changes of ABA content (a) stomatal conductivity (b) in the leaves of Heliotropium thermophilum growing in soil at 20 ± 5 °C, 40 ± 5 °C, 60 ± 5 °C and 80 ± 5 °C. Vertical bars represent standard deviations of the means of three replicates. Different letters denote significant differences among all treatments at P < 0.05
Stomatal conductivity
Stomatal conductivity was highest in plants grown at 40 ± 5 °C. In addition, there was no statistically significant difference between 20 ± 5 °C and 60 ± 5 °C plant groups. Stomatal conductivity was also lowest in plants grown at 80 ± 5 °C (Fig. 3b).
Osmolyte content
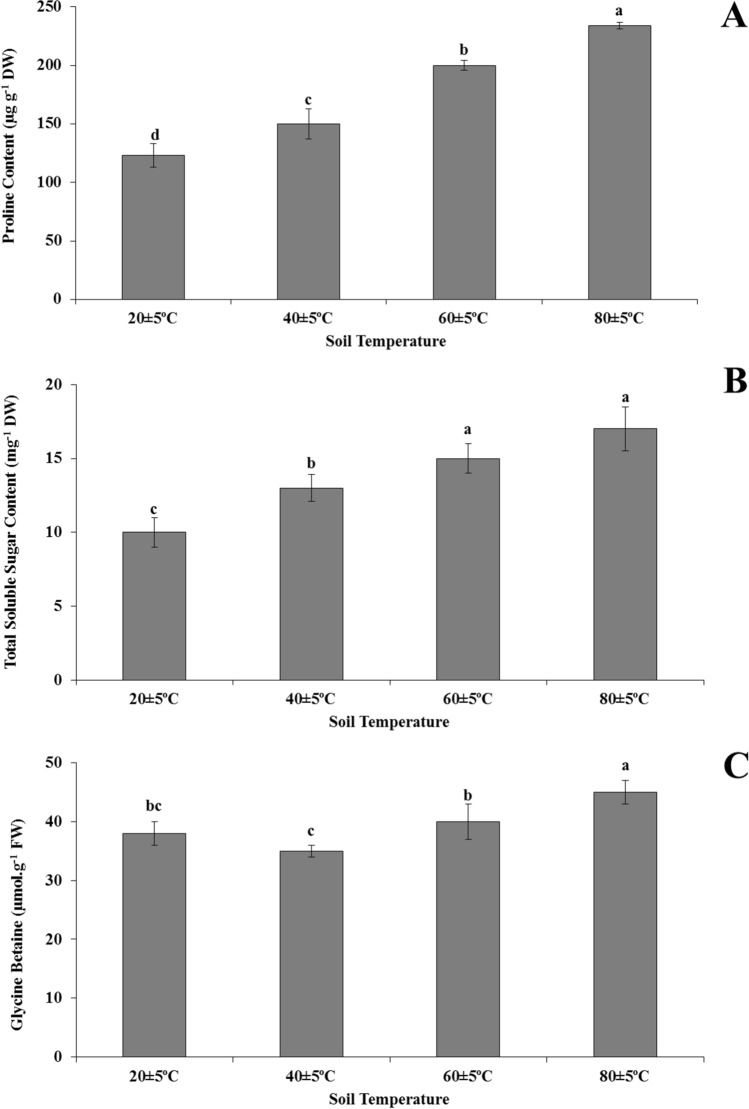
The proline and total soluble sugar contents increased with rising soil temperature compared with the 20 ± 5 °C soil temperature. Highest increases in contents of prolin (1.9-times) and total soluble sugars (1.7-times) were determined in plants grown at 80 ± 5 °C compared to plants growing in soil at 20 ± 5 °C.The glycine betaine levels were not significantly affected from 20 ± 5 °C to 60 ± 5 °C, and increased in the plants grown at at 80 ± 5 °C. In addition, there was highest production of all osmolyte compounds in plants grown at 80 ± 5 °C (Fig. 4).
Fig. 4.
Alterations in organic osmolyte levels, proline (a), total soluble sugar (b), glycine betaine (c) contents in the leaves of Heliotropium thermophilum growing in soil at 20 ± 5 °C, 40 ± 5 °C, 60 ± 5 °C and 80 ± 5 °C. Vertical bars represent standard deviations of the means of three replicates. Different letters denote significant differences among all treatments at P < 0.05
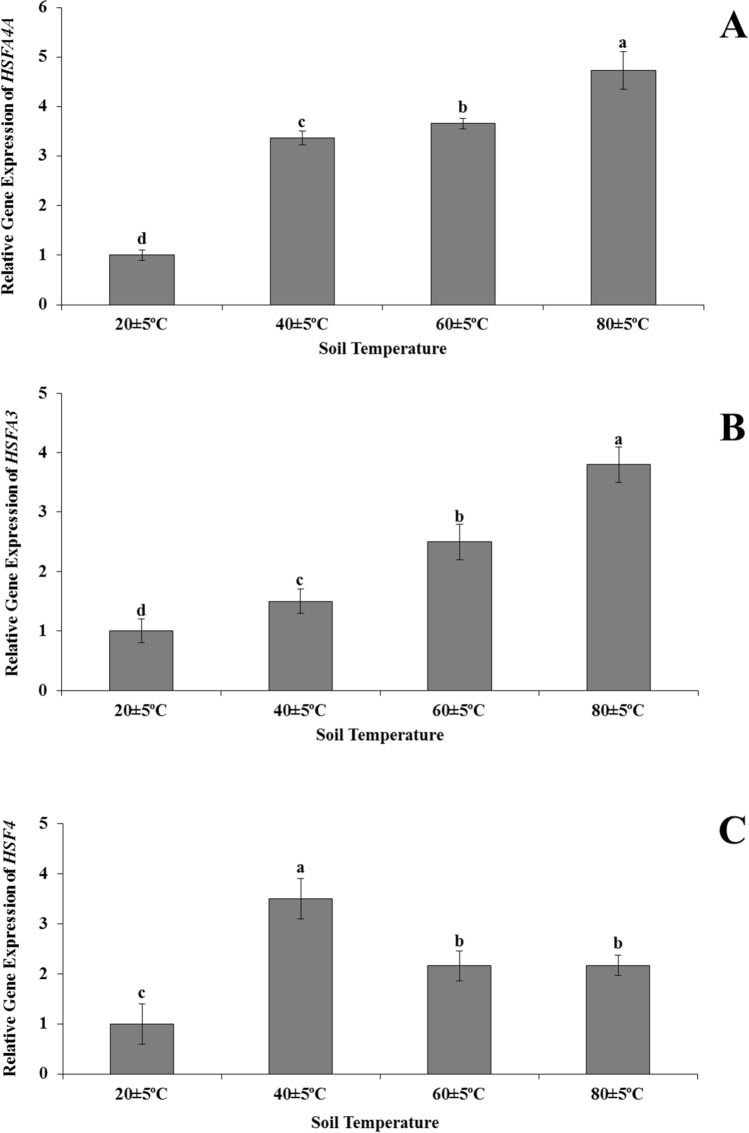
Expression of HSFA4A, HSFA3, and HSF4 genes
The expression levels of HSFA4A and HSFA3 in H. thermophilum at 40 ± 5 °C, 60 ± 5 °C and 80 ± 5 °C were higher compared to H. thermophilum grown at 20 ± 5 °C. Moreover, highest increases in the levels of HSFA4A (4.7-times) and HSFA3 genes (4.0-times) were observed in plants grown at 80 ± 5 °C compared to plants growing in soil at 20 ± 5 °C (Fig. 5 AB). The expression level of the HSF4 gene showed an increase in expression levels of plants at 40 ± 5 °C, 60 ± 5 °C and 80 ± 5 °C compared to plants at 20 ± 5 °C. This increase in HSF4 gene expression was highest in plants at 40 ± 5 °C and there was no significant difference in HSF4 gene expression between plants grown at 60 ± 5 °C and 80 ± 5 °C (Fig. 5c).
Fig. 5.
Alterations in the transcript level of HSFs, HSFA4A (a), HSFA3 (b), HSF4 (c) in the leaves of Heliotropium thermophilum growing in soil at 20 ± 5 °C, 40 ± 5 °C, 60 ± 5 °C and 80 ± 5 °C. Vertical bars represent standard deviations of the means of three replicates. Different letters denote significant differences among all treatments at P < 0.05
Discussion
With the rise of global warming over the last century, the effects of high temperature on physiology, growth, and development of plant need to be better understood in order to maintain agricultural productivity. Therefore, studies on understanding the mechanisms present in thermophilic plants against high temperature conditions are considered important for growing temperature-tolerant plants. Plants are generally exposed to many different stress factors such as fungal, bacterial infections, grazing, drought, temperature changes and salt etc. in their natural habitats. Therefore, studies conducted under controlled laboratory conditions are essential to better understand the thermotolerance mechanisms. It has been reported that H. thermophilum adapted to soil temperatures of 60 ± 5 °C under laboratory conditions (Oztürk et al. 2020). But how this plant develops tolerance mechanism for survival at extremely high soil temperature (80 ± 5 °C) is not yet clear. In the present study, role of ABA, osmolytes and HSFs in thermotolerance to extremely high temperature was investigated for the first time.
Plants under high temperature stress can undergo morphological changes such as leaf senescence and chlorophyll degradation (Allakhverdiev et al. 2008). In our study, there were no such symptoms (such as senescence, color loss in leaves) in H. thermophilum during exposure to extreme heat, but plant growth was observed as reduced (Fig. 1). It has been reported that although obvious visual symptoms were observed in heat-tolerant plants above 35 °C (Xu et al. 2013; Rossi et al. 2017), these symptoms were not observed in H. thermophilum at 60 ± 5 °C and 80 ± 5 °C. Another parameter, TBARS level is one of the most common markers of cellular oxidative damage, due to lipid peroxidation (Savicka and Škute 2010). In the present study, TBARS level (8.36 nmol g−1 fw) in leaves of plant at 80 ± 5 °C was 1.20-times higher compared to plants at 20 ± 5 °C. Previous report in the literature stated that lipid peroxidation (24.03 μmol g−1 fw) in Gossypium hirsutum L. exposed to 45 °C for 2 h was 1.14-times higher compared to the control plants at 30 °C (Gür et al. 2010). Heliotropium thermophilum however, can survive in relatively higher heat stress conditions (80 ± 5 °C). Here we can also say that our result supported the observed morphological changes of plants in present study.
The stress hormone ABA plays a key role in plant defense responses and provides tolerance to abiotic stress conditions (Wang et al. 2017). Heat stress induces a rapid increase in endogenous ABA content, which promotes water balance and strengthens heat tolerance by regulating stomatal closure (Hsieh et al. 2013). The stomata plays a very important role in reducing water loss in plants under various environmental stresses (Osakabe et al. 2014). According to our observations, with increase in soil temperature from 20 ± 5 °C to 80 ± 5 °C, ABA level increased and stomatal conductivity decreased for H. thermophilum, and these two observations may be related.
The accumulation of compatible osmolytes (proline, glycine betaine, soluble sugars, sugar alcohols, and tertiary and quaternary ammonium compounds) in plants is also one of the well-known defense mechanisms in tolerance to high temperature stress (Sairam and Tyagi 2004; Wahid 2007). In our study, proline and total soluble sugar accumulation significantly increased in the plants grown at 40 ± 5 °C, 60 ± 5 °C, and 80 ± 5 °C soil temperature. It has been stated that osmotic regulation is achieved by the accumulation of osmolyte compounds in plants exposed to high temperatures (Sakamoto and Murata 2000). Proline, glycine betaine, and total soluble sugars protect plants from high temperatures by maintaining osmotic homeostasis, buffering cellular redox potential, maintaining membrane stability, or maintaining cell water balance (Farooq et al. 2008). Our observations have shown that the elevated levels of these osmolytes (proline, glycine betaine, and total soluble sugars) can play a role in sustaining water status and maintaining turgor in H. thermophilum grown under high temperature.
Plant transcription factors (TFs), play essential roles in enhancing the stress tolerance of plants including protection from high temperature (Perez-Rodriguez et al. 2010). Plant heat shock factors (HSFs) are among the most important TFs of high heat response in signaling. Many studies have reported on the central roles of HSFs in various abiotic stresses, including high temperature stress (Scharf et al. 2012). In our study, the expression levels of HSFA4A and HSFA3 genes significantly increased with increasing temperature from 20 ± 5 °C to 80 ± 5° C. On the other hand, the expression level of the HSF4 gene was not a statistically significant difference between plants grown at 60 ± 5 °C and 80 ± 5 °C. Although, some studies have shown that some HSFs play a critical role in thermotolerance, others have proved them to play a less critical role (HSP101, HSA32, HSFA1, HSFA3) and to have little effect on the thermotolerance of knockout variants (Larkindale and Vierling 2008; Schramm et al. 2008; Yoshida et al. 2011). Liu and Charng (2013) have showed that in the absence of the HSFA1 transcription factor, a minimal but significant acquired level of thermotolerance may be achieved in Arabidopsis mutants after acclimation, possibly due to the induction of a small number of genes regulated by other transcription factors such as bZIP28.
As mentioned in above results, it was determined that there is a similarity among the changes in HSFs, osmolyte compounds and ABA under high temperatures. The role of abscisic acid, osmolytes and heat shock factors in high temperature thermotolerance of H. thermophilum has been investigated in the present study for the first time.
Conclusions
Our results showed that even though heat stress damages such as senescence and pigment loss in leaves was not observed in plants grown at 20 ± 5 °C, 40 ± 5 °C, 60 ± 5 °C and 80 ± 5 °C, plant growth and development was reduced under high temperatures (60 ± 5 °C and 80 ± 5 °C). Heliotropium thermophilum displays optimal growth at 40 ± 5 °C. Besides, water loss caused by transpiration was prevented by stomatal closing by increased ABA in plants grown under high soil temperatures. Moreover, H. thermophilum have high osmotic adjustment through the accumulation of total soluble sugar, glycine betaine, and especially proline in leaf tissues and can survive in high soil temperatures by promoting the heat tolerance mechanism involving induction of increased transcription level of HSFs. Our observations suggests that H. thermophilum may be extremely thermophilic plant that could survive at extreme soil temperature (80 ± 5 °C) inducing various mechanisms discussed in our study.
Acknowledgements
This study was supported by the project at Karadeniz Technical University Research Projects Unit (Project No: FDK-2017-7214). We also thank to native speaker Russell Fraser for English language improvement.
Authors’ Contributions
ASM and AK designed the research. ASM performed the experiments. ASM and AK analyzed the data and wrote the manuscript. All authors read and approved the manuscript.
Declaration
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Asiye Sezgin Muslu, Email: asiyeszgn@outlook.com.
Asim Kadıoğlu, Email: asimkadioglu@gmail.com.
References
- Allakhverdiev SI, Kreslavski VD, Klimov VV, Los DA, Carpentier R, Mohanty P. Heat stress: an overview of molecular responses in photosynthesis. Photosynth Res. 2008;98:541–550. doi: 10.1007/s11120-008-9331-0. [DOI] [PubMed] [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water stress studies. Plant Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Bita CE, Gerats T. Plant tolerance to high temperature in a changing environment: scientific fundamentals and production of heat stress-tolerant crops. Front Plant Sci. 2013;4:273. doi: 10.3389/fpls.2013.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JL, Klueva NY, Zheng G, Nieto SJ, Ho THD, Nguyen HT. Cloning of new members of heat shock protein HSP101 gene family in wheat (Triticum aestivum (L.) Moench) inducible by heat, dehydration, and ABA. Biochim Biophys Acta. 2001;1517:270–277. doi: 10.1016/S0167-4781(00)00292-X. [DOI] [PubMed] [Google Scholar]
- Ciura J, Kruk J. Phytohormones as targets for improving plant productivity and stress tolerance. J Plant Physiol. 2018;229:32–40. doi: 10.1016/j.jplph.2018.06.013. [DOI] [PubMed] [Google Scholar]
- Cohen Y, Moreshet S, Fuchs M. Changes in hydraulic conductance of citrus trees following a reduction in wetted soil volume. Plant Cell Environ. 1987;10:53–57. doi: 10.1111/j.1365-3040.1987.tb02079.x. [DOI] [PubMed] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Calorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Fitter AH, Hay RKM. Environmental Physiology of Plants. 3. London, UK: Academic Press; 2002. [Google Scholar]
- Farooq M, Basra S, Wahid A, Cheema Z, Cheema M, Khaliq A. Physiological role of exogenously applied glycine betaine to improve drought tolerance in fine grain aromatic rice (Oryza sativa L.) J Agro Crop Sci. 2008;194:325–333. doi: 10.1111/j.1439-037X.2008.00323.x. [DOI] [Google Scholar]
- Gür A, Demirel U, Özden M, Kahraman A, Çopur O. Diurnal gradual heat stress affects antioxidant enzymes, proline accumulation and some physiological components in cotton (Gossypium hirsutum L.) Afr J JBiotechnol. 2010;9:1008–1015. doi: 10.5897/AJB09.1590. [DOI] [Google Scholar]
- Hasanuzzaman M, Nahar K, Alam MM, Roychowdhury R, Fujita M. Physiological, biochemical and molecular mechanisms of heat stress tolerance in plants. Int J Mol Sci. 2013;14:9643–9684. doi: 10.3390/ijms14059643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chloroplast. I. kinetics and stoichiometry of fatty acid peroxidation. Arch Bio Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hsieh EJ, Cheng MC, Lin TP. Functional characterization of an abiotic stress-inducible transcription factor AtERF53 in Arabidopsis thaliana. Plant Mol Biol. 2013;82:223–237. doi: 10.1007/s11103-013-0054-z. [DOI] [PubMed] [Google Scholar]
- Iba K. Acclimative response to temperature stress in higher plants: Approaches of gene engineering for temperature tolerance. Annu Rev Plant Biol. 2002;53:225–245. doi: 10.1146/annurev.arplant.53.100201.160729. [DOI] [PubMed] [Google Scholar]
- Islam MR, Baohua F, Tingting C, Guanfu F. Role of Abscisic acid in thermal acclimation of plants. J Plant Biol. 2018;61:255–264. doi: 10.1007/s12374-017-0429-9. [DOI] [Google Scholar]
- Lange PJ. A revision of the New Zealand Kunzea ericoides (Myrtaceae) complex. Phytokeys. 2014;40:185p. doi: 10.3897/phytokeys.40.7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkindale J, Vierling E. Core genome responses involved in acclimation to high temperature. Plant Physiol. 2008;146:748–761. doi: 10.1104/pp.107.112060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Liu SS, Yi CY, Wang F, Zhou J, Xia XJ, Shi K, Zhou YH, Yu JQ. Hydrogen peroxide mediates abscisic acid-induced HSP70 accumulation and heat tolerance in grafted cucumber plants. Plant Cell Environ. 2014;37:2768–2780. doi: 10.1111/pce.12360. [DOI] [PubMed] [Google Scholar]
- Li Z-G, Yang SZ, Long WB, Yang GX, Shen ZZ. Hydrogen sulfide may be a novel downstream signal molecule in nitric oxide-induced heat tolerance of maize (Zea mays L.) seedlings. Plant Cell Environ. 2013;36:1564–1572. doi: 10.1111/pce.12092. [DOI] [PubMed] [Google Scholar]
- Liu HC, Charng YY. Common and distinct functions of Arabidopsis class A1 and A2 heat shock factors in diverse abiotic stress responses and development. Plant Physiol. 2013;163:276–290. doi: 10.1104/pp.113.221168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng G, Jin-Hong L, Xiao M, De-Xu L, Zhen-Hui G, Ming-Hui Lu. The plant heat stress transcription factors (hsfs): structure, regulation, and function in response to abiotic stresses. Front Plant Sci. 2016;7:114. doi: 10.3389/fpls.2016.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osakabe Y, Osakabe K, Shinozaki K, Tran LP. Response of plants to water stress. Front Plant Sci. 2014;5:86–94. doi: 10.3389/fpls.2014.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oztürk K, Sağlam A, Kadioğlu A. Heliotropium thermophilum, an extreme heat tolerant species, promises plants about adaptation to high soil temperature conditions. Physiol Mol Bio Plants. 2020;26:525–535. doi: 10.1007/s12298-020-00766-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Rodriguez P, Riano-Pachon DM, Correa LG, Rensing SA, Kersten B, Mueller-Roeber B. PlnTFDB: updated content and new features of the plant transcription factor database. Nucleic Acids Res. 2010;38:822–827. doi: 10.1093/nar/gkp805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Burgess P, Jespersen D, Huang B. Heat-induced leaf senescence associated with chlorophyll metabolism in bentgrass lines differing in heat tolerance. Crop Sci. 2017;57:169–178. doi: 10.2135/cropsci2016.06.0542. [DOI] [Google Scholar]
- Sakamoto A, Murata N. genetic engineering of glycine betaine synthesis in plants: current status and implications for enhancement of stress tolerance. J Exp Bot. 2000;51:81–88. doi: 10.1093/jexbot/51.342.81. [DOI] [PubMed] [Google Scholar]
- Sairam R, Tyagi A. Physiology and molecular biology of salinity stress tolerance in plants. Curr Sci. 2004;86:407–421. [Google Scholar]
- Savage MJ, Cass A. Psychrometric field measurement of water potential changes following leaf excision. Plant Physiol. 1984;74:96–98. doi: 10.1104/pp.74.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savicka M, Škute N. Effects of high temperature on malondialdehyde content, superoxide production and growth changes in wheat seedlings (Triticum aestivum L.) Ekologija. 2010;56:26–33. doi: 10.2478/v10055-010-0004-x. [DOI] [Google Scholar]
- Scharf KD, Berberich T, Ebersberger I, Nover L. The plant heat stress transcription factor (Hsf) family: structure, function and evolution. Biochem Biophys Acta. 2012;1819:104–119. doi: 10.1016/j.bbagrm.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Schramm F, Larkindale J, Kiehlmann E, Ganguli A, Englich G, Vierling E, Von Koskull-Döring P. A cascade of transcription factor DREB2A and heat stress transcription factor HsfA3 regulates the heat stress response of Arabidopsis. Plant J. 2008;53:264–274. doi: 10.1111/j.1365-313X.2007.03334.x. [DOI] [PubMed] [Google Scholar]
- Smale M, Fitzgerald N (2015) A field guide to the vegetation associations of the Taupo Volcanic Zone, Landcare Research New Zealand, Waikato Regional Council Technical Report, 32, ISSN 2230-4355.
- Tan K, Çelik A, Gemici Y, Gemici M, Yildirim H. Heliotropium thermophilum (Boraginaceae), a new taxon from SW Anatolia. Turkey Adv Sci Lett. 2008;1:132–139. doi: 10.1166/asl.2008.003. [DOI] [Google Scholar]
- Tercek MT, Hauber DP, Darwin SP. Genetic and historical relationships among geothermally adapted Agrostis (bentgrass) North America and Kamchatka: evidence for apreviously unrecognized thermally adapted taxon. Am J Bot. 2003;90:1306–1312. doi: 10.3732/ajb.90.9.1306. [DOI] [PubMed] [Google Scholar]
- Wahid A. Physiological implications of metabolites biosynthesis in net assimilation and heat stress tolerance of sugarcane (Saccharum officinarum) Sprouts. J Plant Res. 2007;120:219–228. doi: 10.1007/s10265-006-0040-5. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhuang L, Shi Y, Huang B. Up-Regulation of HSFA2c and HSPs by ABA contributing to improved heat tolerance in tall fescue and Arabidopsis. Int J Mol Sci. 2017;18:1981. doi: 10.3390/ijms18091981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Du H, Huang B. Identification of metabolites associated with superior heat tolerance in thermal bentgrass through metabolic profiling. Crop Sci. 2013;53:1626–1635. doi: 10.2135/cropsci2013.01.0045. [DOI] [Google Scholar]
- Yoshida T, Ohama N, Nakajima J, Kidokoro S, Mizoi J, Nakashima K, Maruyama K, Kim JM, Seki M, Todaka D, et al. Arabidopsis HsfA1 transcription factors function as the main positive regulators in heat shockresponsive gene expression. Mol Genet Genomics. 2011;286:321–332. doi: 10.1007/s00438-011-0647-7. [DOI] [PubMed] [Google Scholar]