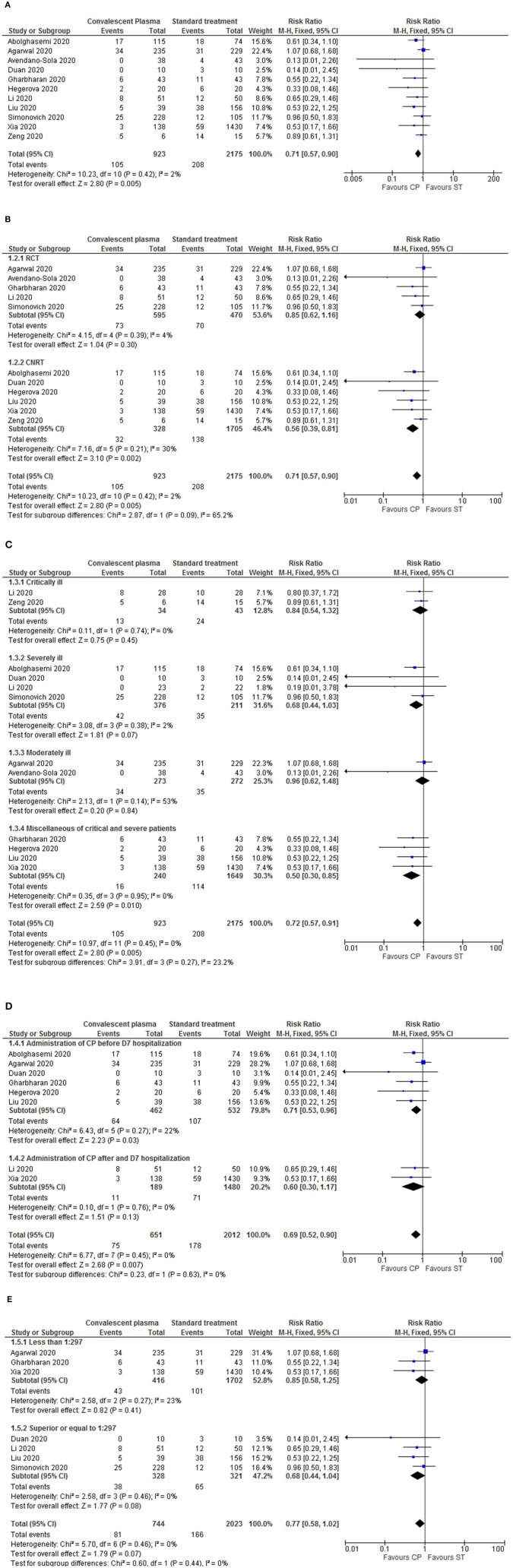
Figure 2.
Forest plots for mortality, and the respective sub-analysis, following CP treatment. (A) For the overall mortality, the results showed a significantly decreased mortality rate in the CP intervention group in comparison to standard treatment. The overall heterogeneity levels are low; therefore, the results for this outcome reported are consistent. Nonetheless, a sensitivity analysis was performed by multiple sub-analysis. (B) Sub-analysis by study design (RCTs vs. CNRTs). (C) Sub-analysis by disease severity (critically, severely, moderately ill and miscallaneous of critical and severe patients). (D) Sub-analysis by administration period of CP (before or after 7 days of hospitalization). (E) Sub-analysis by antibody titers administered (<1:297 or ≥1:297). Different sizes of data markers correspond to the relative weight assigned in the pooled analysis. Diamond marker indicates the overall result.