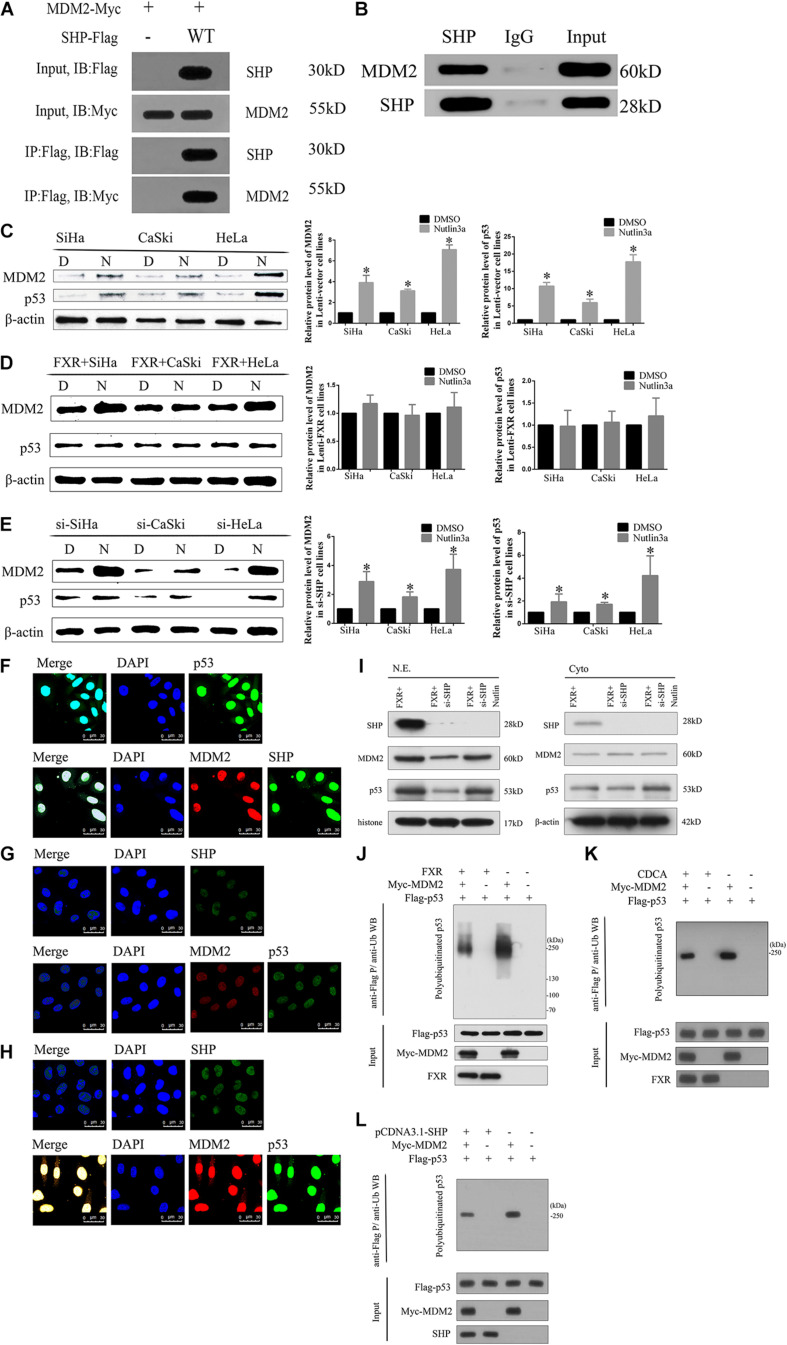
FIGURE 5.
FXR regulates p53 ubiquitination by stabilizing MDM2 aggregation in the nucleus through SHP. (A) Immunoprecipitation and western blot were performed to determine the association of SHP-WT with MDM2. An anti-Myc antibody was used to detect MDM2, and the anti-Flag antibody was used to IP SHP. (B) The MDM2 protein was immunoprecipitated with polyclonal anti-SHP antibodies in Lenti-FXR cells (IP: SHP), and immunoprecipitates were resolved by SDS-PAGE. Immunoblotting was performed with anti-MDM2 antibodies. Protein levels of MDM2 and p53 with or without application of Nutlin-3a were detected by western blot in Lenti-Vector cells (C), Lenti-FXR cells (D), and Lenti-FXR cells transfected with si-SHP (E). D, DMSO; N, Nutlin3a. Immunofluorescent staining of SHP, MDM2, and p53 in SiHa-FXR cells (F) and counterparts co-transfected with si-SHP (G) or si-SHP and Nutlin-3a (H). Nuclei (blue) are stained with 4′-6-diamidino-2-phenylindole (DAPI). (I) Nuclear and cytoplasmic extracts were obtained for immunoblot analysis of MDM2, p53, and SHP expression. (J) SiHa was co-transfected with Flag-p53 and Myc-MDM2; 48 h later, cells were treated with 10 μmol/l MG132 for 2 h and harvested for immunoblot assay with anti-p53 antibody. The SiHa cells were transiently transfected with pCDNA3.1 (K) or cultured in the medium containing CDCA (50 μmol/l) (L). After 48 h, the protein ubiquitination was performed. ∗P < 0.05 vs. DMSO group.