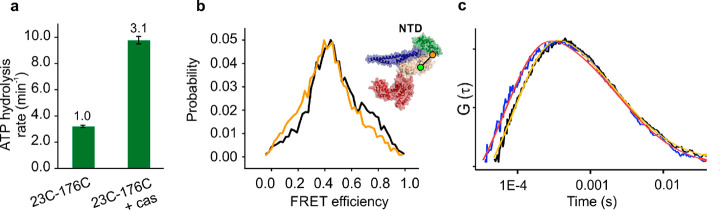
Figure 2.
Analysis of the conformational dynamics of the NTD. (a) ATPase activity of fully labeled 23C-176C protein, either basal or in the presence of 25 μM κ-casein (n = 3). The error bars correspond to standard errors of the mean. The numbers above the bars designate relative activity. (b) FRET efficiency histograms of the 23C-176C mutant of ClpB with a single labeled protomer in a 2 mM ATP solution, either in the absence of substrate–protein (black) or in the presence of 25 μM κ-casein (yellow). Here and elsewhere, FRET efficiency histograms are area-normalized. (c) Filtered FCS cross-correlation curves of 23C-176C ClpB without (black) and with 25 μM κ-casein (blue). The red and yellow curves are fits to eq 1 in the Methods and yield similar diffusion coefficients but faster dynamics in the presence of κ-casein (red) than in its absence (yellow)—see text.