Fig. 1.
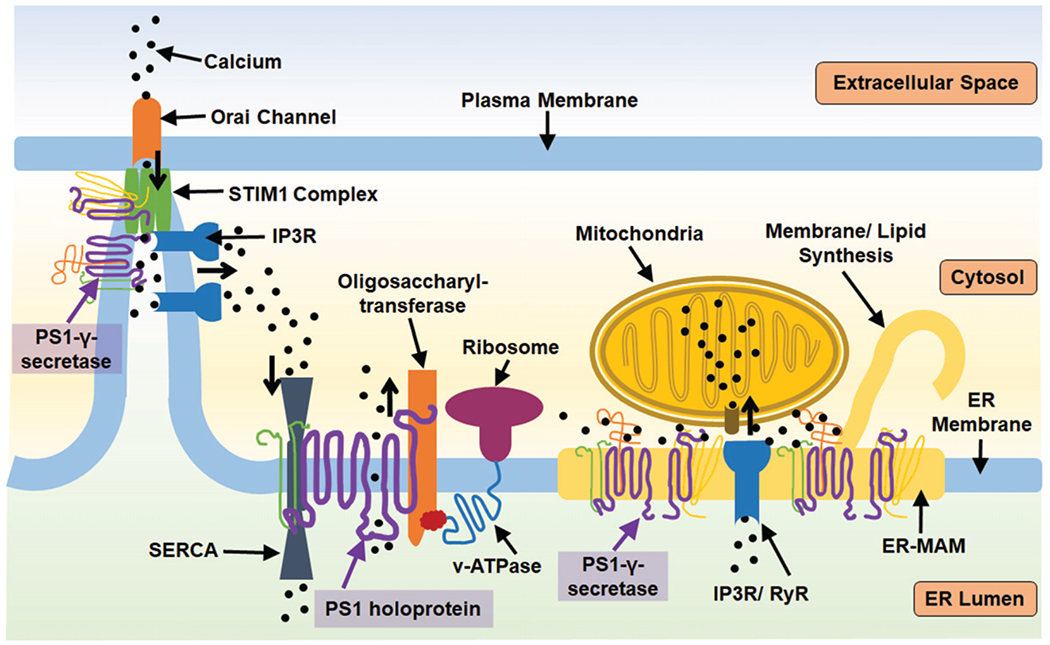
PS1 has multiple roles regulating ER activity both as a holoprotein and as a member of the γ-secretase complex. STIM1 is a calcium sensor that initiates calcium uptake via binding the Orai calcium channel at the plasma membrane-endoplasmic junction when calcium levels are transiently low. PS1 can regulate STIM1 activity by proteolysis, which is enhanced by PS1 mutation. STIM1 function is dependent upon the proper function of IP3R ([IP3 (inositol 1, 4, 5-trisphosphate) receptor] a calcium channel that releases calcium from the ER calcium when activated by IP3 ligand). IP3R is aberrantly active in the context of PS1 mutation. This IP3-STIM1 association is dependent upon ER-mitochondria association and calcium sharing, which is additionally regulated by the presence of PS1 at ER-MAMs. PS1 also regulates the expression and activity of RyR (ryanodine receptor) calcium channels which release calcium from the ER. Additionally, PS1 holoprotein can further impact calcium flux by biding SERCA, sarco/endoplasmic reticulum calcium-ATPase, which pump calcium into the ER, or PS1 may act as a leak channel itself. PS1 holoprotein has demonstrated capacity to aid oligosaccharyltransferase in glycosylation of v-ATPase, demonstrating a means through which PS1 may regulate general ER proteostasis. The role of PS1-γ-secretase at the ER-MAM (endoplasmic reticulum mitochondria associated membrane) can further impact the calcium balance of mitochondria, influencing their oxidative balance, and impact ER-MAM dependent activities such as lipid synthesis.
