Fig. 2.
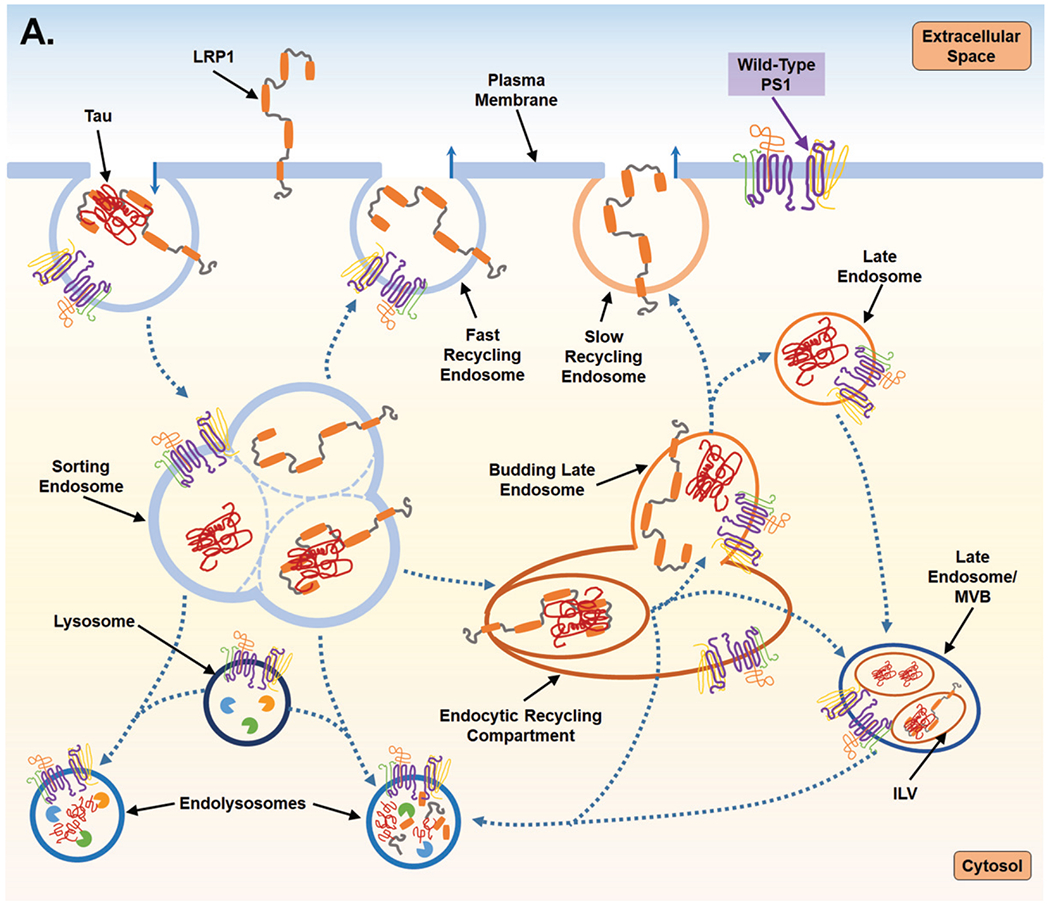
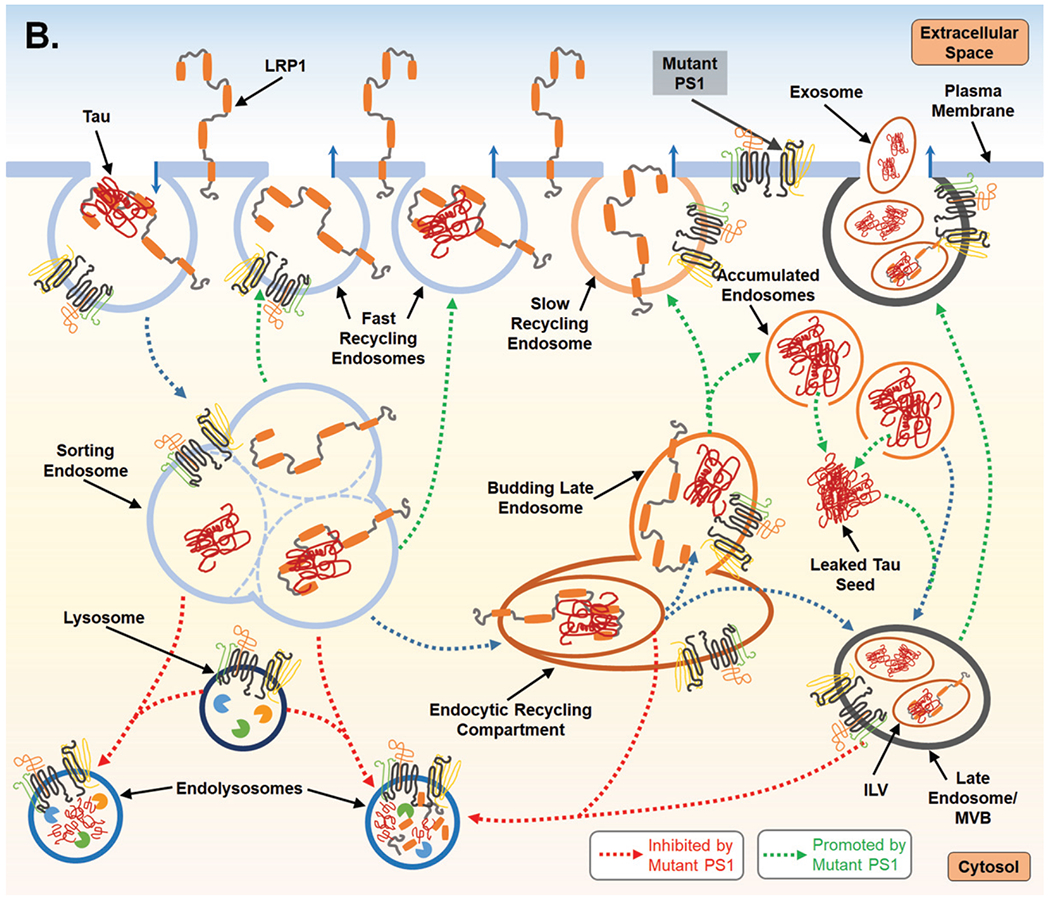
Proposed mechanism for PS1-mediated regulation of endocytosis and the subsequent impact on LPR1 turnover and tau uptake. A) PS1 is present at the cell surface and within multiple vacuolar structures. PS1 is postulated to largely localize within endocytic compartments and regulate their trafficking and fusion. PS1 is also reported to maintain lysosome function which allows for degradation of endocytic cargo after fusion event which forms the endolysosome. LPR1 (low-density lipoprotein receptor-related protein-1) binds tau at the cell surface and is internalized and trafficked to the sorting endosome. At the sorting endosome, LRP1 can be dissociated from tau and recycled to the surface; unbound tau is trafficked toward the functional degradative pathway. Alternatively, tau may remain associated with LRP1 and trafficked to the endocytic recycling compartment (ERC). From the ERC, tau and LRP1 can enter a maturing endosome and dissociate. LRP1 is recycled to the plasma membrane via the slow recycling route, whereas tau is retained in an endosomal compartment and targeted for degradation. As part of steady state LRP1 turnover, associated LRP1 and tau can also be internalized at the ERC into an intra-luminal vesicle (ILV) and trafficked together towards a multivesicular body (MVB), which is also considered a late endosome. From the MVB tau and LRP1 are degraded after fusion with a lysosome. B) Mutant PS1, however, inhibits lysosomal clearance and thus potentially return of LPR1 (with or without tau ligand) to the cell surface from the sorting endosome and from the ERC. Due to inappropriate trafficking, endosomal compartments retain cargo, increasing overall expression of both tau and LRP1. Tau can then leak from endosomal compartments and act as a seed for endogenous, cytosolic tau seen in Alzheimer’s disease. To compensate for disruption of degradative trafficking and vesicular burden, PS1 mutant cells can exocytose tau that has accumulated in MVBs, thereby propagating tau spread.
