Abstract
Lyme and other tick-borne diseases are increasing in the United States. Development of tick control tools have focused primarily on the blacklegged tick, Ixodes scapularis Say. Application of acaricides or entomopathogenic fungal agents to kill host-seeking ticks or ticks on rodents can suppress I. scapularis abundance in residential landscapes, but evidence is lacking for impact on human tick bites or tick-borne disease. Similar studies remain limited for the lone star tick, Amblyomma americanum (L.). Other knowledge gaps include how well homeowners and pest control companies perform in the broadcast application of tick-killing products, relative to high efficacy reported in research studies, and the tick-killing potential of natural product formulations exempt from Environmental Protection Agency registration. Area-wide control based on preventing ticks from feeding on their main reproductive host, the white-tailed deer, can suppress populations of both I. scapularis and A. americanum. Some studies also suggest an impact on Lyme disease cases, but this needs to be further validated in larger-scale intervention studies. The effectiveness, scale, cost, and implementation of various tick management strategies are important considerations in efforts to reduce human tick encounters and tick-borne disease. Additional barriers include weak incentives for industry and academia to develop, test, and register new tick and pathogen control technologies, including vaccines targeting humans, tick reproductive hosts, or wildlife pathogen reservoirs. Solutions will need to be ‘two-pronged’: improving the tick and pathogen control toolbox and strengthening the public health workforce engaging in tick control at local and state levels.
Keywords: Amblyomma, Dermacentor, Ixodes, tick-bite prevention, tick management
The Increasing Problem With Human-Biting Ticks and Tick-Borne Disease in the United States
There has been a continued rise and geographical spread of human-biting ticks and tick-borne disease in the United States (Rosenberg et al. 2018, Sonenshine 2018). This unfortunate reality is forcing the public health community to examine what can be done better to prevent human tick bites and reduce tick-borne illness, in the short-term and into the future (Paules et al. 2018, Beard et al. 2019, Petersen et al. 2019, Rochlin et al. 2019, Eisen 2020). The sections below address key issues for tick management and tick-bite prevention, bearing in mind the following facts:
In some parts of the United States, residents can encounter pathogen-infected ticks in their backyards, while spending time on neighboring properties or in neighborhood green spaces and during professional or recreational activities in other areas (Hahn et al. 2016, Stafford et al. 2017, Mead et al. 2018, Fischhoff et al. 2019a).
The responsibility for tick control and tick-bite prevention currently falls on individuals, because organizational structure for local tick management is either lacking or poorly developed across the United States (Piesman and Eisen 2008, Eisen 2020).
Tick management is becoming increasingly complex as major human-biting species, with differing host and habitat preferences, expand their ranges and state and local public health entities are forced to contend with multiple human-biting tick vector species (Stafford 2007, Eisen et al. 2016, Sonenshine 2018, Jordan and Egizi 2019, Molaei et al. 2019).
In the near future, tick management and tick-bite prevention will, by necessity, be a ‘two-pronged’ strategy where most of the responsibility continues to be shouldered by individuals as professionally staffed tick management programs are developed. Unless the integrated tick management (ITM) approaches adopted by such programs truly have area-wide spatial coverage, one important issue moving forward will be to delineate the responsibility among management programs and individual homeowners to suppress ticks on privately owned properties, which are considered important settings for exposure to ticks infected with Lyme disease spirochetes in the Northeast (Falco and Fish 1988, Maupin et al. 1991, Stafford and Magnarelli 1993, Stafford et al. 2017, Mead et al. 2018, Fischhoff et al. 2019a). Moreover, the availability and use of current and new products for tick and pathogen management are dependent upon the interactions among public health entities and research institutions, commercial development, public acceptance, and actual implementation of various tick management tools by homeowners and pest management professionals (Fig. 1).
Fig. 1.
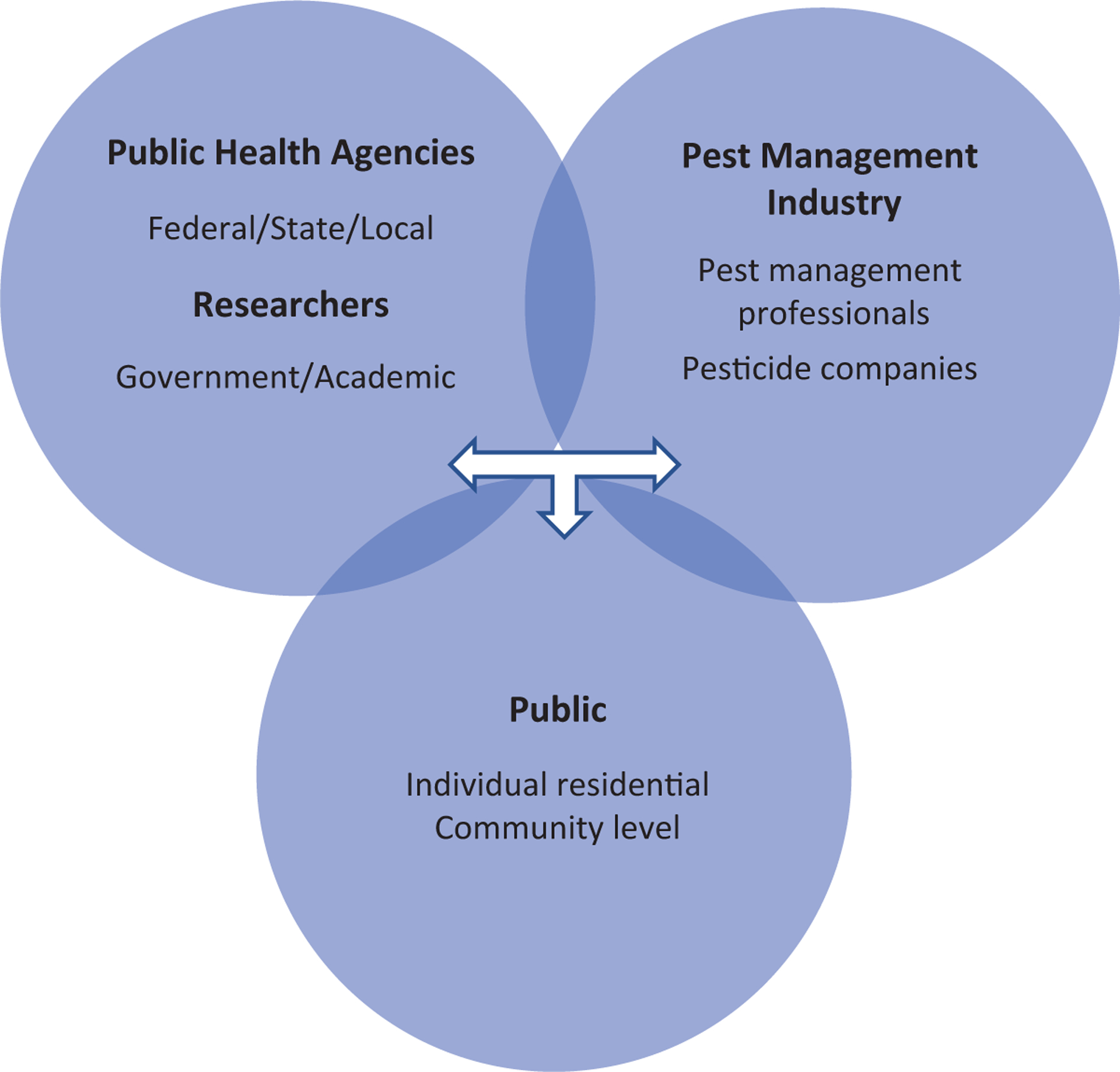
The tick management triad responsible for research, commercial development, and implementation of strategies for the management of ticks and reduction of tick-borne diseases (commercial development is not necessarily restricted to the pest management industry).
To discuss the general topic at hand in some depth, we have chosen to focus on one increasingly common scenario in the eastern United States where humans locally are at risk for exposure to the blacklegged tick, Ixodes scapularis Say, the American dog tick, Dermacentor variabilis (Say), and the lone star tick, Amblyomma americanum (L.) (Acari: Ixodidae) (Rossi et al. 2015, Xu et al. 2016, Jordan and Egizi 2019). These three tick species collectively transmit at least 14 human pathogens, including viral disease agents (Bourbon virus, Heartland virus, and Powassan virus); bacterial agents causing Lyme disease (Borrelia burgdorferi sensu stricto [s.s.] and Borrelia mayonii), relapsing fever (Borrelia miyamotoi), anaplasmosis (Anaplasma phagocytophilum), ehrlichiosis (Ehrlichia chaffeensis, Ehrlichia ewingii, Ehrlichia muris eauclairensis, and Panola Mountain Ehrlichia), spotted fever rickettsiosis (Rickettsia rickettsii), and tularemia (Francisella tularensis); and a parasite causing babesiosis (Babesia microti) (Jellison 1974; Burgdorfer 1977, 1984; Spielman et al. 1985; Childs and Paddock 2003; Paddock and Yabsley 2007; Ebel 2010; Eisen et al. 2017; Eisen and Eisen 2018; Lehane et al. 2020; Telford and Goethert 2020). For general overviews of other notable human-biting ixodid tick vectors in the United States—including Ixodes pacificus Cooley and Kohls, Dermacentor andersoni Stiles, Dermacentor occidentalis Marx, Amblyomma maculatum Koch, and Rhipicephalus sanguineus sensu lato—we refer to previous publications (James et al. 2006; Dantas-Torres 2010; Paddock and Goddard 2015; Eisen et al. 2016, 2017; Padgett et al. 2016; Paddock et al. 2018).
Adapting General Integrated Pest Management Principles for ITM and Setting Program Goals
Bearing in mind the increasing complexity of the threat posed by ticks and tick-borne pathogens in the United States, no single environmentally based method (Table 1) may suffice to counter this broad threat when implemented in a manner that is acceptable both in terms of cost and environmental impact. The only single environmentally based control method capable of substantially reducing the abundance of all three major human-biting ticks in the eastern United States (A. americanum, D. variabilis, and I. scapularis) is broadcast of acaricides (synthetic or natural product-based formulations) or biological control agents (entomopathogenic fungi) to kill host-seeking ticks. However, repeated area-wide broadcast of acaricides across a range of tick habitats at large scales simply is not environmentally responsible. Even spatially targeted broadcast on single residential properties can be challenging, because we have yet to define a spatio-temporal application scheme that can be expected to reduce both the abundance of host-seeking ticks and human tick encounters across residential properties. As a case in point, a research study that evaluated a low-cost spatio-temporal application scheme using a minimal amount of synthetic acaricide on residential properties (a single annual [late spring] application restricted to the grass-woods interface, covering an unknown portion of the actual tick-encounter risk habitat present on the properties) showed moderate 45–69% suppression across years for the abundance of host-seeking I. scapularis nymphs, specifically within the treated habitat, but no reduction for encounters of the residents with ticks (Hinckley et al. 2016). The outcome of this research study raised the yet unanswered questions of whether reduction of human tick bites could have been achieved by a more aggressive temporal application scheme, as typically employed in real-world acaricide applications by pest control companies (Schulze et al. 1997, Stafford 1997, Jordan and Schulze 2019a), and/or broadcast application covering the full extent of residential high-risk tick habitats, as now attempted in the ‘Tick Project’ using a biological control agent (Keesing and Ostfeld 2018). A more intensive broadcast application is guaranteed to be more costly to the homeowner and, depending on the specific type of product used, also may lead to unwanted negative environmental impacts such as collateral damage to backyard pollinators (Ginsberg et al. 2017). Another question to be answered is how proficient are homeowners and commercial pest control operators in applying broadcast acaricides or biological control agents so that they reach the vast majority of host-seeking ticks present within the treated area. Efficient tick-killing requires an understanding not only of the product formulation and application equipment used but also of the behavior of host-seeking ticks.
Table 1.
Characteristics of tick-bite prevention and tick/pathogen management methods in relation to key human-biting ticks in the eastern United States (Ixodes scapularis, Amblyomma americanum, and Dermacentor variabilis)
| Tick-bite prevention or tick/pathogen management method | Impact on tick species or associated pathogens | Spatial extent of protection | Temporal extent of protection | Lag time from first use to protection | |||
|---|---|---|---|---|---|---|---|
| I. scapularis | A. americanum | D. variabilis | |||||
| Personal protective measure | |||||||
| Tick check (and removal of attached ticks) | Yes | Yes | Yes | Unlimited | Day (when used) | None | |
| Repellents or permethrin-treated clothing | Yes | Yes | Yes | Unlimited | Day (when used) | None | |
| Human anti-tick vaccinea | TBDc | TBD | TBD | Unlimited | TBD (likely 1–3 yr) | TBD (likely months) | |
| Human Lyme disease vaccinea | Yesd | No | No | Unlimited | TBD (likely 1–3 yr) | TBD (likely months) | |
| Landscape/vegetation management | |||||||
| Xeriscaping or hardscapingb | Yes | Yes | Yes | Landscaped area | Permanent | None | |
| Vegetation management | Yes | Yes | Yes | Managed area | Months to yearsb | Weeks to months | |
| Remove rodent harborage | Yes | No | Yes | Rodent home rangef | One to several yearsb | 1 yr | |
| Deer fencing | Yes | Yes | No | Fenced area | Permanent for intact fence | 1–2 yr | |
| Killing of host-seeking ticks | |||||||
| Acaricide | Yes | Yes | Yes | Treatment area | 1–2 mo (single application) | 1 d | |
| Entomopathogenic fungal agent | Yes | Uncleare | Uncleare | Treatment area | ≤1 mo (single application) | 1–2 wk | |
| Rodent-targeted method | |||||||
| Topical acaricide (or oral acaricidea) | Yes | No | Yes | Rodent home rangef | 1 yr | 1 yr | |
| Rodent anti-tick vaccinea | Yes | No | Yes | Rodent home rangef | 1 yr | 1 yr | |
| Oral rodent Lyme disease vaccinea | Yesd | No | No | Rodent home rangef | 1 yr | 1 yr | |
| Deer-targeted method | |||||||
| Deer reduction | Yes | Yes | No | Deer home rangeg | Years | 1–3 yri | |
| Topical acaricide (or oral acaricidea) | Yes | Yes | No | Deer home rangeg | Years | 1–3 yri | |
| Deer anti-tick vaccinea | TBD | TBD | No | Deer home rangeg | Years | 1–3 yri | |
Not yet available for public use.
Including xeriscape/hardscape barrier along the lawn/woods ecotone.
TBD, to be determined.
Impact on Borrelia burgdorferi sensu stricto infection but not on infection with other pathogens associated with I. scapularis or tick density.
Field studies on the impact of entomopathogenic fungal agents on this tick species are still lacking from the published literature.
Variable across habitats, rodent species, and sexes but typically in the range of 0.1 to 1.0 ha for white-footed mice.
Variable across habitat types and sexes but in the range of 10 to >100 ha for white-tailed deer in urban settings.
Depending on the action (e.g., mowing, leaf litter removal, brush clearing, or removal of rodent harborage) and how often it needs to be repeated.
1–2 yr for A. americanum but 2 3 yr for I. scapularis.
To reach the goal of reducing human tick bites and preventing human disease, while at the same time minimizing negative impacts on the environment, it is instructive to consider how long-established principles for Integrated Pest Management (IPM) can be adapted for management of human-biting ticks. The objectives of classic IPM are to 1) prevent unacceptable levels of pest damage; 2) minimize the risk to people, property, infrastructure, natural resources, and the environment; and 3) reduce the evolution of pest resistance to pesticides and other pest management practices (USDA 2018). This involves the selection, integration, and implementation of several pest control actions based on predicted ecological, economic, and sociological consequences. Adapting the IPM concept to address human-biting ticks and tick-borne disease in an ITM program could involve the following rephrased goals: 1) prevent unacceptable levels of human tick bites and tick-borne disease; 2) minimize the risk posed by the intervention to people, domestic animals, and the environment; and 3) prevent the development of resistance in the targeted tick species to acaricides or other control agents (Ginsberg and Stafford 2005). It also should be noted that acceptable levels of risk for human tick encounters may be very different for recreational areas (higher tolerance) and residential areas (very low tolerance).
Although the goal of classic IPM is to prevent unacceptable levels of pest damage, management, unlike control, implies an acceptable level of pest abundance and acceptable level of damage or loss below an economic injury level, i.e., the pest abundance at which the losses exceed management costs. In our case, this could be balancing the management costs associated with controlling ticks with the social and medical costs of Lyme and other tick-borne diseases (i.e., a cost–benefit analysis), or simply the cost of reducing human tick encounters to some acceptable level of risk (i.e., a cost-efficacy or cost-effectiveness analysis) (Ginsberg and Stafford 2005). Although it may be feasible to calculate and contrast tick management costs, as recently done for the use of rodent-targeted topical acaricides on residential properties (Jordan and Schulze 2019b), with the direct and indirect costs of the number of tick-borne disease cases prevented (Mac et al. 2019), robust assessments require yet unavailable data on the impact of single and ITM approaches on the occurrence of disease cases. An alternative cost–benefit assessment approach could target the projected risk for human tick encounters. For example, Mount and Dunn (1983) proposed an economic injury level, to avoid recreation and tourism losses, of 0.65 A. americanum ticks per 1-hr carbon dioxide trap operation, which based on an observed relationship between trap catch and human tick encounters predicted an attack rate of less than 1 tick per human visitor per day in the examined recreational area.
In the current scenario where homeowners are directly responsible for controlling ticks on their properties, either doing it themselves with over-the-counter products or hiring a pest control company, willingness to pay is a major concern. A study from Connecticut conducted in 2002–2004 revealed that the majority of homeowners were unwilling to spend more than $100 per year to control ticks on their properties (Gould et al. 2008), which should be viewed in relation to that the typical amount charged by pest control companies per acre for a single application of synthetic or natural product acaricide is $150–200 (Jordan and Schulze 2019a). Additional issues contributing to low levels of homeowners taking action to control ticks on their properties, or to use personal protective measures for family members and pets, include unwillingness to use acaricides and repellents, especially synthetic formulations, and limited knowledge of other options, such as rodent-targeted acaricides and biological control agents (Gould et al. 2008, Hook et al. 2015, Jordan and Schulze 2019a, Niesobecki et al. 2019).
From a broader, societal perspective, key issues to consider in reducing human tick encounters and tick-borne diseases include effectiveness, scale, and cost, together with countering barriers to implementation and understanding what the ultimate goal should be: control, elimination, or eradication. For tick management programs, the goals for interventions have not been well defined, particularly as a reduction in either human tick bites or human disease has been difficult to document. Definitions, linguistic uncertainty, and associated expectations matter in IPM (Cira et al. 2019). Control, management, reducing risk, and elimination are all terms that have been used in exploring ‘tick control’. Efforts at reducing malaria provide a comparison and have been defined as control, elimination, and eradication (Hall and Fauci 2009). One key difference between malaria and tick-borne disease to keep in mind is that the reservoir host for malaria parasites is people, whereas tick-borne pathogens are zoonotic agents with wildlife reservoir hosts. Malaria control is defined as the reduction of disease incidence, prevalence, morbidity, or mortality to a locally acceptable level. Continued intervention is required to sustain control. This concept of control probably best fits the current state of our efforts to combat human-biting ticks and tick-borne disease.
Malaria elimination is the interruption of local transmission (i.e., reducing the rate of malaria cases to zero) in a defined geographic area. Continued intervention measures are required to prevent the re-establishment of transmission. Elimination of a tick-borne disease agent as a threat to humans can conceptually be achieved in different ways: 1) suppressing the tick population to levels where human tick encounters no longer occur; 2) suppressing the tick population to levels that interrupt enzootic pathogen transmission, thus rendering ticks infrequent nuisance biters of humans rather than vectors of disease agents; or 3); eliminating the pathogen from the population of vertebrate reservoirs via vaccination or antimicrobial treatment, which again would render ticks human nuisance biters rather than vectors of disease agents but without reducing tick numbers. The level of difficulty to achieve elimination varies across tick-borne pathogens, being most likely to succeed in scenarios that involve tick species and pathogens with narrow host and reservoir ranges and most challenging in scenarios where the tick vector can utilize a wide range of host animal species and many of these also serve as pathogen reservoirs. Moreover, elimination likely is most feasible in isolated settings, such as physical or ecological islands, with limited potential for continued influx of ticks and vertebrates from surrounding areas. Perhaps the best example of an intervention that approached elimination is the complete removal of white-tailed deer (Odocoileus virginianus) from Monhegan Island off the coast of Maine. This action prevented I. scapularis from completing its life cycle on the island, by removing the host for the adult tick stage, and reduced the presence of this tick to low numbers of host-seeking nymphal and adult specimens considered to have resulted from fed ticks of the preceding life stages continually introduced to the island by migrating birds (Rand et al. 2004, Elias et al. 2011).
Malaria eradication is a permanent reduction to zero of world-wide infection. Realistically, eradication at a global scale is not possible with a zoonotic vector-borne disease without the virtual eradication of the reproductive or pathogen reservoir hosts, similar to what happened in colonial times in the northeastern United States when white-tailed deer (the primary reproductive host for I. scapularis) were essentially eradicated and forests were cleared for agriculture. The emergence of Lyme disease in the 1970s and the continuing rise of this massive public health threat has been linked with reforestation and increased populations of white-tailed deer (Barbour and Fish 1993, Spielman 1994); the resurgence of deer populations also have been linked to increased populations of A. americanum (Paddock and Yabsley 2007). From the tick vector perspective, the goal of ITM programs would be to reduce tick abundance, infection prevalence, or human tick encounters to levels resulting in reduced numbers of tick-borne disease. Using Lyme disease as the example of a tick-borne disease, program goals could be to prevent all Lyme disease cases locally (essentially elimination under the malaria program definition) or to reduce cases below some arbitrary threshold where it is no longer considered a local public health issue (falling under control based on the malaria program definition).
For tick-borne diseases, any part of the triad of host, vector, and pathogen and their interactions within the local environment can be the target of control interventions (Table 1). One of the most intriguing problems with an ITM approach is the optimal selection of methods to integrate in order to maximize the reductions in abundance of host-seeking infected ticks and human tick encounters on single properties or across larger areas. Single intervention methods can impact host-seeking ticks, ticks on hosts, or infection in vertebrate reservoirs, and there is a lack of empirical data on which combinations of such methods most effectively will suppress tick abundance or disrupt enzootic pathogen transmission cycles (Ginsberg 2001, Ginsberg and Stafford 2005, Eisen et al. 2012, Stafford et al. 2017). The published literature includes data for the outcomes of many individual environmentally based control methods to suppress host-seeking ticks and infection in ticks (reviewed by Eisen and Dolan 2016, White and Gaff 2018), and for a few ITM approaches targeting I. scapularis (Schulze et al. 2007, 2008; Eisen and Dolan 2016; Stafford et al. 2017; Williams et al. 2018a, b). Some earlier work on ITM examined combinations of acaricide spraying, vegetation management, and deer management (fencing) for the control of A. americanum in a recreational setting (Bloemer et al. 1990). However, there is a critical lack of empirical data from studies specifically designed to assess improved tick and pathogen suppression in relation to increased cost for integration of multiple control methods versus each method used individually. Moreover, as noted previously (Eisen and Gray 2016, Stafford et al. 2017), published data are still lacking for the impact of any ITM approach on human tick bites and tick-borne disease, and very few ongoing studies are even attempting to generate such data (Keesing and Ostfeld 2018, Connally 2020). In the following sections, we examine the efficacy of existing tick management methods and the rationale, barriers, and potential solutions to tick management at the residential and area-wide scales, tick-bite prevention, and industry engagement.
Impact of Existing Tick Management and Tick-Bite Prevention Methods Across Spatial Scales for Human-Biting Tick Species (I. scapularis, D. variabilis, and A. americanum)
In a complex risk scenario with local occurrence of multiple human-biting tick species, it is important not only to understand when and where you are at risk for bites by the different life stages of these ticks, but also to what extent different tick-bite prevention and tick management methods can be expected to reduce the risk of tick encounters. These methods can vary in impact across spatial scales, and some methods may not be applicable to the biology of all individual human-biting tick species (Table 1). For example, rodent-targeted methods would not be applicable for A. americanum, as the immature stages do not readily utilize rodent hosts (Zimmerman et al. 1987, Allan et al. 2010), and deer-targeted methods would not be applicable for D. variabilis, as deer are not favored hosts by this tick species (Cooney and Burgdorfer 1974, Kollars et al. 2000). Some single intervention methods have very narrow impacts across the spectrum of ticks and their associated pathogens (e.g., vaccination of mice against B. burgdorferi s.s., without impact on other I. scapularis-borne pathogens for which these animals also serve as reservoirs, including A. phagocytophilum and B. microti), whereas other methods have potential to broadly impact all tick species (broadcast application of acaricides or biological control agents, and use of tick repellents). Moreover, the spatial extent of protection can range from the treated area along a lawn-woods interface in the backyard (barrier spraying of acaricides or biological control agents) to the entire backyard (rodent-targeted acaricides and deer fencing), the larger landscape (deer reduction and deer-targeted acaricides), or be scale-free (personal protective measures, including repellents and permethrin-treated clothing, and in the future hopefully also a new Lyme disease vaccine for use in humans). As indicated in Table 1, it is important to also consider the length of time that a given method affords protection after being implemented and if there is a lag time from implementation to protection.
Broadcast applications of conventional synthetic acaricides are highly effective against I. scapularis when done correctly with high penetration of the tick microhabitat (Eisen and Dolan 2016), and represent the most common tick management method currently used by pest control companies to treat backyards (Jordan and Schulze 2019a). Although similar broadcast application may be feasible at larger geographic scales, for example via aircraft, synthetic acaricides are unlikely to be implemented in such a manner due to various environmental concerns including the impact on non-target organisms such as pollinators and the increased potential for the development of acaricide resistance (Ginsberg and Stafford 2005, Piesman and Eisen 2008, Ginsberg et al. 2017). Natural product-based acaricides or entomopathogenic fungal agents may be more acceptable for large scale use but have less robust killing efficacy profiles against I. scapularis (more sensitive to environmental conditions) and shorter duration of efficacy requiring more frequent applications (Eisen and Dolan 2016). Recent studies on broadcast of conventional synthetic acaricides (pyrethroids) have demonstrated efficacy against both I. scapularis and A. americanum (Jordan et al. 2017, Schulze and Jordan 2019), whereas similar studies for currently available synthetic pesticides labeled for use against ticks are lacking for D. variabilis.
Rodent-targeted acaricides have the benefit of the source pesticide being confined to delivery devices, rather than being spread openly in the environment, and all rodent-targeted approaches (acaricides, vaccines, or anti-microbials) impact an area defined by the home ranges of the treated animals. For example, the white-footed mouse, Peromyscus leucopus—which is an important reservoir host for B. burgdorferi s.s., B. mayonii, B. miyamotoi, B. microti, and A. phagocytophilum—is a territorial species with reported home ranges averaging 0.1 to 1 ha in different environments (Wolff 1985, Nupp and Swihart 1996, Gaitan and Millien 2016). Two different products for topical application of acaricide to rodents are currently on the market: the Damminix or Thermacell Tick Tube, where permethrin-treated cotton balls intended for use as nesting material are offered to rodents in cardboard tubes, and the SELECT Tick Control System (TCS), where rodents are treated with fipronil as they navigate toward a food bait in a bait box (Eisen and Dolan 2016). A recent study comparing these products indicated that SELECT TCS is more effective in preventing tick bites across both mice and chipmunks, but that the Damminix Tick Tube holds the advantage for both availability (only the Tick Tubes can be purchased over-the-counter by homeowners) and implementation cost (10-fold lower for the Damminix Tick Tube when used by homeowners) (Jordan and Schulze 2019b). However, another yet unpublished larger-scale study found no significant impact of a SELECT TCS intervention on either host-seeking I. scapularis nymphs or human tick encounters (Hinckley et al. 2018).
Similar recent studies are lacking for D. variabilis, but Sonenshine and Haines (1985) found that a bait box treating rodents with an insecticidal dust or oil reduced the numbers of immatures of this species on the rodent hosts. Moreover, a new formulation of an oral rodent-targeted vaccine against B. burgdorferi s.s. is showing promise in field trials (Stafford et al. 2020) with high bait acceptance by white-footed mice (Williams et al. 2020). One important consideration for rodent-targeted approaches is that the impact on acarological risk for human exposure to I. scapularis nymphs will not be evident until the year after the intervention is put in place. For example, implementing a rodent-targeted topical acaricide or vaccine in the spring and summer of Year 0 will not result in reductions to the abundance of host-seeking I. scapularis nymphs, or the prevalence of infection with B. burgdorferi s.s. in the nymphs, until the spring of Year 1.
White-tailed deer—the main reproductive host for I. scapularis and A. americanum—have large-scale individual home ranges, from 8 to 42 ha for does and 28–130 ha for bucks in urban areas (reviewed by Stewart et al. 2011, Stafford and Williams 2017). Population reduction or acaricide treatment of white-tailed deer, therefore, can impact large areas. These two approaches have been associated with reduction in the abundance of host-seeking I. scapularis (reviewed by Eisen and Dolan 2016, Stafford and Williams 2017, Telford 2018), and, in some cases, also reduction of Lyme disease cases (Garnett et al. 2011, Kilpatrick et al. 2014), although with some caveats (Kugeler et al. 2016). The main complication with deer reduction is lack of acceptability for reducing deer to the very low densities required to substantially reduce I. scapularis and A. americanum populations, potentially followed by the interruption of enzootic pathogen transmission cycles. Topical application of tick-killing pesticides on white-tailed deer, using U.S. Department of Agriculture 4-poster deer treatment stations, is better accepted and has resulted in significant reductions in the abundance of host-seeking I. scapularis and A. americanum at the community level in several studies, with the caveat that the success of this intervention is related to an adequate density of deployed 4-poster devices (reviewed by Eisen and Dolan 2016, Stafford and Williams 2017). Deployment of 4-poster devices is currently limited by municipal and state regulations due to concerns regarding the corn food bait used to attract deer to the devices and the open access to pesticide on the rollers used to self-treat the deer as they reach in to eat the corn. Specific issues include label restrictions on deployment near residences or places where children may be present, which can require a safety fence around the 4-poster device; feeding of deer and attraction of non-target wildlife, such as raccoons and bears; and increased risk for spread of diseases impacting deer, such as chronic wasting disease and bovine tuberculosis. A final consideration for population reduction or acaricide treatment of white-tailed deer is a multi-year lag time before these interventions reach peak impact on host-seeking I. scapularis and A. americanum ticks.
Published studies on ITM approaches for I. scapularis are scarce but have shown promising results to suppress ticks infesting rodents or host-seeking ticks when combining SELECT TCS rodent bait boxes with the broadcast of entomopathogenic fungus (Met52, containing Metarhizium anisopliae) (Williams et al. 2018a, b), or when these two approaches were combined with 4-poster deer treatment stations (Schulze et al. 2007, 2008). An earlier ITM study focusing on A. americanum (Bloemer et al. 1990) showed strong suppression in the abundance of host-seeking ticks using a combination of vegetation management (mowing, leaf litter removal, and selective removal of overstory and midstory vegetation), deer fencing and broadcast of synthetic pesticide (the organophosphate chlorpyrifos, which presently is available for use against host-seeking ticks, including A. americanum, only in certain settings, including golf course turf, road medians, and turf and ornamentals around industrial buildings).
ITM on Single Residential Properties: Rationale, Barriers, and Solutions
As tick management remains an individual responsibility, homeowners need to take action to protect family members from acquiring tick bites on their property. The question then becomes how to decide what to do and how to implement personal protective measures (discussed in the section ‘Tick-bite prevention through personal protective measures: rationale, barriers, and solutions‘) and environmentally based tick suppression methods. The best solution for each property will depend on its physical attributes combined with the ability/willingness to pay and what the family deems acceptable methods to use. One main barrier is for the homeowner to gather all the relevant information needed on tick biology and tick suppression methods, from reliable web-based information sources or pest control companies, to be able to make an informed decision. The home environment has long been recognized as an important setting for exposure to I. scapularis in the Northeast (Falco and Fish 1988, Maupin et al. 1991, Stafford and Magnarelli 1993, Stafford et al. 2017, Mead et al. 2018), and increasingly also for A. americanum as this tick’s range has expanded northward along densely populated areas of the Eastern Seaboard (Springer et al. 2014, Stafford et al. 2018, Jordan and Egizi 2019, Molaei et al. 2019). As not all tick suppression methods designed for use against I. scapularis also are effective against A. americanum (Table 1), it is important for the homeowner to understand which tick species are present around the home before deciding on which tick suppression methods to invest in. Options for tick suppression methods that can be used on a single property include:
Deer fencing to prevent deer from entering the property and depositing fed ticks, including female ticks which can lay thousands of eggs.
Hardscaping and xeriscaping to reduce the amount of suitable tick habitat on the property.
Vegetation management, including mowing to reduce the amount of grassy area with high humidity favoring tick host-seeking and survival; tree, brush, and leaf litter removal to further reduce the portion of the property with optimal (shady and moist) tick microhabitat conditions; and selection of plants not favored by deer.
Other landscaping to reduce the time family members spend in or near to high-risk tick habitat on the property, and to remove rodent harborage such as wood piles or rock walls.
Rodent-targeted acaricides to prevent I. scapularis immatures from feeding on pathogen reservoirs (no impact on A. americanum or its associated pathogens).
Killing of host-seeking ticks via broadcast of conventional synthetic acaricides, natural product-based acaricides, or entomopathogenic fungal control agents.
As noted in recent publications (Eisen and Dolan 2016, Stafford et al. 2017, Fischhoff et al. 2019b), the empirical evidence for each of these potential components of an ITM approach to reduce the abundance of host-seeking ticks when implemented singly on residential properties ranges from more robust (killing of host-seeking ticks, use of rodent-targeted acaricides, and deer fencing) to weaker (brush and leaf litter removal) and very limited or lacking (landscaping, mowing, and plant selection). Intriguing findings from recent experimental studies include suppression of I. scapularis adults due to management of invasive brush (Williams et al. 2017) and increased abundance of host-seeking I. scapularis nymphs associated with accumulation of leaves from leaf blowing or raking activities (Jordan and Schulze 2020), but there is a continued need to better understand the impact of these and other landscaping and vegetation management approaches on host-seeking ticks on residential properties. Moreover, empirical data for the impact of ITM approaches on host-seeking ticks on single residential properties remain restricted to the combination of rodent-targeted acaricide and broadcast of an entomopathogenic fungal agent (Williams et al. 2018a, b). One ongoing study (Connally 2020) that combines rodent-targeted acaricide and barrier spraying of synthetic acaricide on single versus multiple adjacent properties aims to understand the impact of this ITM approach on both host-seeking I. scapularis and human tick encounters, but results are pending. Another major ongoing ITM study (Keesing and Ostfeld 2018) that combines rodent-targeted acaricide and broadcast of entomopathogenic fungal agents, and includes impacts on host-seeking I. scapularis and human tick encounters as well as tick-borne illness, is focused on the neighborhood scale and it is not clear how the results from large groups of neighboring properties will translate to implementation on a single property surrounded by non-treated properties. There is a critical need for new studies to explore how deer fencing, landscaping, vegetation management, and killing of host-seeking ticks or ticks on hosts can best be combined as single property ITM approaches that are both acceptable for use and economically viable. Knowledge of the extent to which landscaping and vegetation management contribute to reduce the abundance of host-seeking ticks and human tick encounters on residential properties is especially weak (Eisen and Dolan 2016).
One important but still not adequately understood issue is the spatial extent of protection within a property provided by a given ITM approach in relation to how the residents use the protected versus unprotected portions of the property. The issues of variable acarological risk for tick exposure across different habitats on residential properties and the impact of specific human behaviors to modify the likelihood of contacting host-seeking ticks were previously addressed in depth by Eisen and Eisen (2016). Rodent-targeted approaches, including acaricide treatment and vaccination (Richer et al. 2014, Schulze et al. 2017, Stafford et al. 2020), and deer fencing can readily be implemented to protect the entire area of smaller residential properties. In contrast, broadcast application of acaricides to kill host-seeking ticks often focuses on the grass-woods ecotone (barrier application of sprays or granular formulations) and therefore may impact only a portion of the wooded high-risk habitat for tick encounters present on the property (Stafford 2007, Hinckley et al. 2016, Jordan and Schulze 2019a). It is, therefore, critically important that the residents not only understand which portions of their properties pose high risk for exposure to ticks but also which specific areas received treatment when a pest control company was contracted for broadcast application of acaricide. Another major barrier to creating tick-free backyards is limited homeowner acceptability for use of synthetic acaricides and low willingness to pay for tick control (Gould et al. 2008, Hook et al. 2015). This commonly results in homeowners not taking action to suppress ticks on their properties in Lyme disease-endemic areas (Gould et al. 2008, Connally et al. 2009, Hook et al. 2015, Niesobecki et al. 2019). Low willingness to pay for tick control is perhaps best addressed via education campaigns specifically targeting options for protection against ticks in the peridomestic environment, including costs and benefits, but it must be noted that there are limits to what can be achieved if most homeowners are unwilling to spend more than $100 per year on tick control (Gould et al. 2008). Limited acceptability for broadcast application of synthetic acaricides may potentially be overcome by use of natural product-based acaricides, biological control agents such as entomopathogenic fungal agents, and rodent-targeted acaricides or vaccines contained in deployment devices (Niesobecki et al. 2019, Jordan and Schulze 2019a). One caveat is that the ongoing proliferation of minimum risk natural products marketed for tick control but exempt from registration by the Environmental Protection Agency (EPA), the ‘25b exempt products’, appear to include some formulations with poor tick-killing efficacy (Dyer 2016). A recent survey of pest control and landscaping companies in New Jersey, New York, and Pennsylvania (Jordan and Schulze 2019a) revealed that the most common tick control methods used on residential properties include application of conventional synthetic pesticides (offered by 80% of survey respondents), application of natural or organic products (34%), habitat management (24%), and host-targeted devices such as Damminix Tick Tubes (22%). Most of these tick control actions were driven by client requests and presence of perceived tick habitat, whereas results of tick surveys only rarely were used as a basis to decide whether or not to treat a client’s property. Moreover, the majority of companies claimed to be unfamiliar with entomopathogenic fungal tick control products and SELECT TCS bait boxes for topical acaricide application to rodents (Jordan and Schulze 2019a). A final point to keep in mind is that our current tick suppression efficacy estimates for different control methods on residential properties are based on research studies (reviewed by Eisen and Dolan 2016), and there is a very poor understanding of how those same methods perform in the hands of either homeowners or pest control companies contracted for tick control. As tick control requires an understanding of tick biology as well as pesticide formulations and application equipment, it would not be surprising to find that pest control companies and, especially, homeowners are less effective in suppressing ticks compared to the expectations for tick-killing efficacy emerging from research studies.
Area-Wide ITM Programs: Rationale, Barriers, and Solutions
Although residential properties in suburban/exurban settings of the Northeast remain important locations for human tick exposure, the ongoing spread and population increase of I. scapularis and A. americanum across the northern part of the eastern United States may result in a more spatially diffuse risk for tick encounters as the abundance of host-seeking ticks reach levels across the landscape where even activities of limited duration (compared to the time spent on your own property) increasingly results in tick encounters. Area-wide ITM approaches may have protective spatial extents ranging in size from neighborhoods to large tracts of public land. Potential methods to integrate for area-wide suppression of ticks include some of those mentioned previously for single residential properties—deer fencing (for smaller target areas), vegetation management (including trail edge treatments; McKay et al. 2020), killing of host-seeking ticks, and rodent-targeted approaches—but also reduction of deer populations and topical acaricide application to deer. Another consideration is whether the ITM approach aims to: 1) suppress ticks broadly across the landscape; 2) focus on specific settings within the landscape, on private or public land, considered to have especially high potential for human tick encounters; or a combination of 1) and 2) for maximum impact. Due in large part to lack of local tick management programs staffed with public health professionals, the concept of area-wide ITM is groundbreaking in the United States and new models for this approach need to be developed and evaluated (Eisen 2020).
Across the primary tick species addressed in this Forum paper, perhaps the best examples of published research studies fitting the concept of area-wide ITM are the efforts by Bloemer et al. (1990) to suppress A. americanum in a campground portion of a recreational area through a combination of deer fencing, vegetation management, and broadcast of acaricide in campgrounds; and the work by Schulze et al. (2007, 2008) to suppress I. scapularis across a mosaic of private and public land through a combination of 4-poster devices for acaricide application to deer, bait boxes for acaricide application to rodents, and barrier broadcast of synthetic acaricide on residential properties to provide interim protection during the lag-period before the rodent-targeted intervention component could be expected to impact host-seeking I. scapularis nymphs. This latter strategy is intriguing as it presents a staggered approach where a less environmentally friendly method without a time-lag for impact on I. scapularis nymphs (broadcast of acaricide) was used as a short-term solution for protection of residential properties until the more environmentally friendly host-targeted methods took effect on residential properties (rodent-targeted acaricide) or across the landscape (deer-targeted acaricide). An ongoing ITM study aiming to suppress I. scapularis have replaced the broadcast of acaricide with an entomopathogenic fungal agent to further improve the environmental profile of the intervention (K. C. Stafford, unpublished data). Another ongoing study is aiming to provide area-wide suppression of I. scapularis at the neighborhood scale without targeting deer but rather treating a substantial portion of the residential properties within the neighborhood with a combination of broadcast of an entomopathogenic fungal agent and rodent-targeted acaricide, potentially also extending some protection to non-treated properties bordering treated properties (Keesing and Ostfeld 2018). The best example of a successful area-wide ITM program targeting a human-biting tick and a tick-borne disease in the United States is the effort to suppress R. sanguineus sensu lato and Rocky Mountain spotted fever on native American land through a combination of peridomestic broadcast of a conventional synthetic acaricide, use of acaricide-treated dog collars, and dog population control, thus attacking the tick both on its favored host and while off-host (Drexler et al. 2014).
Compared to suppression of ticks from backyard-to-backyard across the nation, area-wide ITM with potential for landscape-wide impact is a goal well worth striving for. However, we will not reach this goal without scaling up the efforts to overcome barriers and answer several critical questions. The white-tailed deer should be at the center of efforts to design area-wide ITM approaches (Stafford and Williams 2017, Telford 2018) but new, societally acceptable technologies are needed to prevent I. scapularis and A. americanum from feeding on this crucial host not only in recreational areas but also in neighborhood settings. Another critical need is to evaluate the potential for the most promising area-wide ITM approaches to impact not only host-seeking ticks but also human tick encounters and tick-borne disease, which requires rigorous and very costly studies. A final consideration is that area-wide ITM programs, should they show promise to reduce human tick bites and human illness, likely will require an increased public health workforce at the local level for their implementation and oversight.
Tick-Bite Prevention Through Personal Protective Measures: Rationale, Barriers, and Solutions
There is little doubt that human-biting ticks will remain part of our future and will require use of personal protective measures against tick bites and pathogen transmission. Tick repellents represent an affordable solution with proven efficacy against bites by the major human-biting tick species and with immediate and scale-free protection. But they are only protective when used, and therein lies the crux for those who live in places where ticks can be encountered in the backyard, along the neighborhood trail where the dog is walked, along the edges of school grounds and sports fields, and while hiking in local recreational areas on the weekends. This need for constant vigilance probably is part of the explanation for the low level (typically 20–40% across studies) of use of tick repellents even in New England and Mid-Atlantic states where Lyme disease is highly endemic (Gould et al. 2008, Vazquez et al. 2008, Connally et al. 2009, Hook et al. 2015, Butler et al. 2016, Gupta et al. 2018, Niesobecki et al. 2019). In the above set of studies, daily tick checks were reported more commonly, but were still not done consistently by more than half of respondents. Because use of personal protective measures (e.g., repellents, permethrin-treated clothing, and tick checks) rely on self-application every time individuals venture into outdoor environments where ticks may be present, such strategies may not reliably impact tick-borne disease incidence in a human population.
Limited understanding of the specific circumstances under which human tick encounters most commonly occur presents another problem for use of burdensome personal protective measures. Factors to consider include: 1) whether tick encounters occur most commonly in the peridomestic environment or in other areas (Falco and Fish 1988, Stafford et al. 2017, Mead et al. 2018, Fischhoff et al. 2019a, Jordan and Egizi 2019); 2) how specific human behaviors may increase the risk of coming into contact with host-seeking ticks in different habitat types (Carroll and Kramer 2001, Lane et al. 2004, Eisen and Eisen 2016); and 3) the time spent in different settings and engaging in specific activities (U.S. Bureau of Labor Statistics 2017, Mead et al. 2018). To make more effective use of personal protective measures, further research is needed to clarify how specific human activities conducted in defined habitats on residential properties or during recreational activities on public lands relate to risk for tick encounters. Another poorly understood issue is where on the human body ticks representing different species and life stages make first contact, knowledge of which could inform how tick repellents and permethrin-treated clothing should be optimally used.
Human vaccines to disrupt tick feeding and/or prevent pathogen transmission have the distinct benefits of requiring only occasional application (e.g., annual booster shots) and protection against the ticks people fail to discover before they attach and have a chance to start transmitting pathogens. No such vaccines are currently available for use in humans against any North American tick species or tick-borne pathogen, although there are several canine Lyme disease vaccines on the market. Effective human vaccines are available for tick-borne encephalitis virus (TBEV) in Europe and Russia, but a recent increase in tick-borne encephalitis cases in Europe illustrates the difficulty in maintaining sufficient vaccination rates (Süss 2008, Erber et al. 2018), as also seen recently in the measles outbreak in some communities in the United States (Patel et al. 2019). For Lyme disease in the United States, an application of an early model to assess various intervention strategies in a hypothetical community found that the use of a Lyme disease vaccine and the application of acaricides to deer produced the greatest reduction in human cases of Lyme disease under best-case scenarios (Hayes et al. 1999); two methods, not incidentally, that have broad-scale or scale-free impacts. However, the best-case scenario for the vaccine was 95% usage in the hypothetical community. The first human Lyme disease vaccine was pulled off the market in 2002 over concerns about adverse reactions and low public acceptance (Schuijt et al. 2011, Shen et al. 2011, Embers and Narasimhan 2013, Steere and Livey 2012), and we have now waited for nearly two decades for a human Lyme disease vaccine to reemerge. At present, one new candidate Lyme disease vaccine (VLA15; Valneva, Lyon, France) has progressed to phase 2 clinical trials in Europe and the United States. There also is considerable interest in anti-tick vaccines, with potential for blocking transmission of multiple pathogens transmitted by the same or multiple tick species (de la Fuente 2018, Rego et al. 2019). However, the potential for such anti-tick vaccines to emerge as public health products is still unclear.
Industry Engagement: Rationale, Barriers, and Solutions
Industry has three broad roles in the field of tick control and tick-bite prevention: production of already marketed products to kill or repel ticks; commercial development of new acaricide or repellent active ingredients and formulations, and other novel products such as anti-tick vaccines or reservoir-targeted transmission-blocking vaccines; and the services provided by pest management professionals (i.e., local pest control companies or franchises). The market, obviously, drives much of industry research and development (Graf et al. 2004). Coincidentally, most pesticide products labeled for environmental tick control also are used for a variety of other arthropod pests. Not surprisingly, there is a wide range of tick and flea control products for companion animals, encompassing flea/tick collars, topical sprays, topical spot-on solutions, and a newer class of oral parasiticides, the isoxazolines (Blagburn and Dryden 2009, Pfister and Armstrong 2016). Among these are more than 30 brand name products, not counting generics, containing at least 16 active ingredients, not counting growth regulators in the formulations, specifically targeting fleas (Stafford 2017). People love their pets, which are often thought of as members of the family (Charles and Davies 2008, Johnson 2009). A recent survey in Connecticut and Maryland found that 83% of respondents used tick control products for pets (Niesobecki et al. 2019). These pet products also may have human health implications as pet ownership can increase the risk of encountering ticks (Jones et al. 2018). Moreover, long-acting tick collars (with flumethrin and imidacloprid) were an important tool in a successful community approach to control brown dog ticks and prevent Rocky Mountain spotted fever (Drexler et al. 2014).
One basic problem is getting industry to invest in developing new products or translating research into commercial products for an unclear public health tick control market. The implementation of novel technologies requires long-term research followed by commercial product development with associated costs, patent or licensing issues, registration approvals, marketing, and actual acceptance and use by the public or pest management professionals (Graf et al. 2004). Nevertheless, there are some products on the market, or close to the market, that were developed for commercial application: examples include devices to treat rodents or deer with topical acaricides, and a rodent reservoir-targeted vaccine against Lyme disease spirochetes. For the majority of such products, federal funding helped move them through initial proof-of-concept studies and early-stage efficacy evaluations in the laboratory and field. One example of such a process is nootkatone from Alaskan yellow cedar and grapefruit essential oil: this compound was shown to be both repellent and toxic to I. scapularis and A. americanum in a series of federally funded laboratory and field studies (Panella et al. 1997, 2005; Dietrich et al. 2006; Dolan et al. 2007, 2009; Behle et al. 2011; Flor-Weiler et al. 2011, Jordan et al. 2011, 2012; Anderson and Coats 2012; Bharadwaj et al. 2012). Nootkatone is commonly used in foods and fragrances, and, very importantly, can be mass-produced using a yeast fermentation process to reduce production cost to the point where nootkatone-based products to repel and kill ticks should be commercially viable. Such nootkatone-based products are being developed under the name ‘NootkaShield’ by Evolva under license from the Centers for Disease Control and Prevention, and registration of nootkatone as a biopesticide is, at the time of this writing, under review by the U.S. Environmental Protection Agency (Evolva 2020). Another similar example is a reservoir-targeted oral bait to vaccinate rodents against B. burgdorferi s.s. (Gomes-Solecki et al. 2006; Richer et al. 2011, 2014; Stafford et al. 2020), developed by U.S. Biologic and at the time of this writing under regulatory review by the U.S. Department of Agriculture (US Biologic 2020). One common problem illustrated by these two examples is the long time (nearly 15 yr) that elapsed from the initial proof-of-concept studies to even nearing product registration and marketing. Shortening the time for development of novel tick and pathogen control products is a critical need for effective future intervention programs. The development of new products combining currently available technology such as oral systemic acaricides, reservoir-targeted vaccines, and different delivery systems under patent by different companies is another potential barrier to industry engagement, the synergism of existing technologies, and the emergence of new products. There also may be regulatory obstacles for product concepts that involve several Federal regulatory agencies, depending on the product and required registrations and approvals.
Tick control services for private residents is offered by pest management professionals (licensed pesticide applicators), with the majority (80%) of surveyed companies in Pennsylvania, New Jersey, and New York offering stand-alone tick control programs (Jordan and Schulze 2019a), an increase over that noted previously in Connecticut (51%) (Stafford 1997). As illustrated in previous (Schulze et al. 1997, Stafford 1997) and the latest (Jordan and Schulze 2019a) survey of pest control companies, the industry continues to utilize synthetic acaricides for tick control. Bifenthrin was the primary acaricide used by over half the survey respondents, followed by cyfluthrin and deltamethrin. Cedar oil was the principal ‘natural product’ used, which raises some concerns over yet undocumented efficacy; none reported using the commercially available entomopathogenic fungus M. anisopliae. Moreover, as pointed out by Jordan and Schulze (2019a): ‘an IPM approach for tick control does not easily fit a pest control model’ within existing pest control company business practices and what clients are willing to pay for tick control services. ITM for residential properties might require further public-private partnerships that would encourage commercial development of effective, affordable tick control products and expedite the development and implementation of existing and/or promising new intervention technologies.
Conclusions
The widest diversity and most current tools for tick control have been developed for I. scapularis, with less recent research on control methods for A. americanum and especially for D. variabilis. Studies have shown that the application of acaricides or entomopathogenic fungal agents to kill host-seeking and ticks on rodents can suppress I. scapularis in the residential landscape, but substantial reductions in the abundance of host-seeking ticks thus far has shown little to no documented impact on human tick bites or human disease. There are limited studies on the efficacy of current acaricides and biopesticides for control of A. americanum in residential settings. One main barrier is our poor understanding of target thresholds for tick suppression at the local (residential), municipal, and state levels to impact the incidence of Lyme and other tick-borne diseases. Similarly, analogous to vaccination thresholds needed for ‘herd’ protection, it is unknown at what level of participation residential (e.g., percent household participation) and community-wide (e.g., percentage and spatial distribution of protected areas within the community) implementation of tick management interventions may result in reduced human tick bites or tick-borne illness. Another knowledge gap is how well homeowners and pest control companies really perform in effective broadcast application of acaricides, relative to the high expectations for killing efficacy set for the same products and application methods in research studies. The major issues in reducing human tick encounters and the incidence of human tick-borne disease can be summed up in terms of effectiveness, scale, cost, and implementation of various management strategies in the tick control toolbox. Development and testing of technologies should be added to that list. In other words, the barriers to area-wide ITM are technical, logistical, and social. Solutions might include intensified research and development by the government and public/private partnerships of existing and novel tick management technologies; support for the commercialization and marketing of the most promising existing technologies and/or new technologies; enhanced education of the public on the efficacy, use, and availability of tick management strategies; and a major increase in the public health workforce engaging in tick control at local and state levels to complement and assist with current efforts driven by homeowners and pest control companies.
Acknowledgments
The members of the ‘Tick Biology, Ecology, and Control’ Subcommittee of the 2020 Tick-Borne Disease Working Group provided stimulating discussions around the topic addressed in this forum paper.
Footnotes
Publisher's Disclaimer: Disclaimer: The findings and conclusions of this study are by the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. The mention of commercial products does not represent an endorsement by the authors or the Centers for Disease Control and Prevention.
References Cited
- Allan BF, Goessling LS, Storch GA, and Thach RE. 2010. Blood meal analysis to identify reservoir hosts for Amblyomma americanum ticks. Emerg. Infect. Dis 16: 433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JA, and Coats JR. 2012. Acetylcholinesterase inhibition by nootkatone and carvacrol in arthropods. Pestic. Biochem. Physiol 102: 124–128. [Google Scholar]
- Barbour AG, and Fish D. 1993. The biological and social phenomenon of Lyme disease. Science. 260: 1610–1616. [DOI] [PubMed] [Google Scholar]
- Beard CB, Visser SN, and Petersen LR. 2019. The need for a national strategy to address vector-borne disease threats in the United States. J. Med. Entomol 56: 1199–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behle RW, Flor-Weiler LB, Bharadwaj A, and Stafford KC III. 2011. A formulation to encapsulate nootkatone for tick control. J. Med. Entomol 48: 1120–1127. [DOI] [PubMed] [Google Scholar]
- Bharadwaj A, Stafford KC III, and Behle RW. 2012. Efficacy and environmental persistence of nootkatone for the control of the blacklegged tick (Acari: Ixodidae) in residential landscapes. J. Med. Entomol 49: 1035–1044. [DOI] [PubMed] [Google Scholar]
- Blagburn BL, and Dryden MW. 2009. Biology, treatment, and control of flea and tick infestations. Vet. Clin. North Am. Small Anim. Pract 39: 1173–1200, viii. [DOI] [PubMed] [Google Scholar]
- Bloemer SR, Mount GA, Morris TA, Zimmerman RH, Barnard DR, and Snoddy EL. 1990. Management of lone star ticks (Acari: Ixodidae) in recreational areas with acaricide applications, vegetative management, and exclusion of white-tailed deer. J. Med. Entomol 27: 543–550. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W 1977. Tick-borne diseases in the United States: Rocky Mountain spotted fever and Colorado tick fever. A review. Acta Trop 34: 103–126. [PubMed] [Google Scholar]
- Burgdorfer W 1984. Discovery of the Lyme disease spirochete and its relation to tick vectors. Yale J. Biol. Med 57: 515–520. [PMC free article] [PubMed] [Google Scholar]
- Butler AD, Sedghi T, Petrini JR, and Ahmadi R. 2016. Tick-borne disease preventive practices and perceptions in an endemic area. Ticks Tick Borne Dis. 7: 331–337. [DOI] [PubMed] [Google Scholar]
- Carroll JF, and Kramer M. 2001. Different activities and footwear influence exposure to host-seeking nymphs of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae). J. Med. Entomol 38: 596–600. [DOI] [PubMed] [Google Scholar]
- Charles N, and Davies CA. 2008. My family and other animals: pets as kin. Sociological Res. Online 13. http://www.socresonline.org.uk/13/15/14.html [Google Scholar]
- Childs JE, and Paddock CD. 2003. The ascendancy of Amblyomma americanum as a vector of pathogens affecting humans in the United States. Annu. Rev. Entomol 48: 307–337. [DOI] [PubMed] [Google Scholar]
- Cira TM, Quick K, and Venette RC. 2019. Watch your language: consequences of linguistic uncertainty for insect management. Am. Entomol 65: 258–267. [Google Scholar]
- Connally NP 2020. The Backyard Integrated Tick Managment (BITM) Study. https://tickipmwg.files.wordpress.com/2018/08/connally_ipm_mar2018-publish.pdf (accessed 10 January 2020).
- Connally NP, Durante AJ, Yousey-Hindes KM, Meek JI, Nelson RS, and Heimer R. 2009. Peridomestic Lyme disease prevention—Results of a population-based case-control study. Am. J. Prev. Med 37: 201–206. [DOI] [PubMed] [Google Scholar]
- Cooney JC, and Burgdorfer W. 1974. Zoonotic potential (Rocky Mountain spotted fever and tularemia) in the Tennessee Valley region. I. Ecologic studies of ticks infesting mammals in Land Between the Lakes. Am. J. Trop. Med. Hyg 23: 99–108. [DOI] [PubMed] [Google Scholar]
- Dantas-Torres F 2010. Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasit. Vectors 3: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich G, Dolan MC, Peralta-Cruz J, Schmidt J, Piesman J, Eisen RJ, and Karchesy JJ. 2006. Repellent activity of fractioned compounds from Chamaecyparis nootkatensis essential oil against nymphal Ixodes scapularis (Acari: Ixodidae). J. Med. Entomol 43: 957–961. [DOI] [PubMed] [Google Scholar]
- Dolan MC, Dietrich G, Panella NA, Montenieri JA, and Karchesy JJ. 2007. Biocidal activity of three wood essential oils against Ixodes scapularis (Acari: Ixodidae), Xenopsylla cheopis (Siphonaptera: Pulicidae), and Aedes aegypti (Diptera: Culicidae). J. Econ. Entomol 100: 622–625. [DOI] [PubMed] [Google Scholar]
- Dolan MC, Jordan RA, Schulze TL, Schulze CJ, Manning MC, Ruffolo D, Schmidt JP, Piesman J, and Karchesy JJ. 2009. Ability of two natural products, nootkatone and carvacrol, to suppress Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) in a Lyme disease endemic area of New Jersey. J. Econ. Entomol 102: 2316–2324. [DOI] [PubMed] [Google Scholar]
- Drexler N, Miller M, Gerding J, Todd S, Adams L, Dahlgren FS, Bryant N, Weis E, Herrick K, Francies J, et al. 2014. Community-based control of the brown dog tick in a region with high rates of Rocky Mountain spotted fever, 2012–2013. PLoS One 9: e112368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer MC 2016. Evaluating the effect of minimal risk natural products for control of the tick, Ixodes scapularis. Open Access Master’s Theses, Paper 952, University of Rhode Island. http://digitalcommons.uri.edu/theses/952 [Google Scholar]
- Ebel GD 2010. Update on Powassan virus: emergence of a North American tick-borne flavivirus. Annu. Rev. Entomol 55: 95–110. [DOI] [PubMed] [Google Scholar]
- Eisen L 2020. Stemming the rising tide of human-biting ticks and tick-borne diseases, United States. Emerg. Infect. Dis 26: 641–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen L, and Dolan MC. 2016. Evidence for personal protective measures to reduce human contact with blacklegged ticks and for environmentally based control methods to suppress host-seeking blacklegged ticks and reduce infection with Lyme disease spirochetes in tick vectors and rodent reservoirs. J. Med. Entomol 53: 1063–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen L, and Eisen RJ. 2016. Critical evaluation of the linkage between tick-based risk measures and the occurrence of Lyme disease cases. J. Med. Entomol 53: 1050–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, and Eisen L. 2018. The blacklegged tick, Ixodes scapularis: an increasing public health concern. Trends Parasitol. 34: 295–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen L, and Gray J. 2016. Lyme borreliosis prevention strategies: United States versus Europe, pp. 429–450. In Braks MAH, van Wierner SE, Takken W, and Sprong H (eds.), Ecology and prevention of Lyme borreliosis. Wageningen Academic Publishers, Wageningen, The Netherlands. [Google Scholar]
- Eisen RJ, Eisen L, and Beard CB. 2016. County-scale distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the continental United States. J. Med. Entomol 53: 349–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Piesman J, Zielinski-Gutierrez E, and Eisen L. 2012. What do we need to know about disease ecology to prevent Lyme disease in the northeastern United States? J. Med. Entomol 49: 11–22. [DOI] [PubMed] [Google Scholar]
- Eisen RJ, Kugeler KJ, Eisen L, Beard CB, and Paddock CD. 2017. Tick-borne zoonoses in the united states: persistent and emerging threats to human health. ILAR J. 58: 319–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias SP, Smith RP Jr,Morris SR, Rand PW, Lubelczyk C, and Lacombe EH. 2011. Density of Ixodes scapularis ticks on Monhegan Island after complete deer removal: a question of avian importation? J. Vector Ecol 36: 11–23. [DOI] [PubMed] [Google Scholar]
- Embers ME, and Narasimhan S. 2013. Vaccination against Lyme disease: past, present, and future. Front. Cell. Infect. Microbiol 3: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erber W, Schmitt H-J, and Vuković Janković T. 2018. Epidemiology by country—An overview, pp. 114–127. In Dobler G, Erber W, and Schmitt H-J (eds.), Tick-borne Encephalitis (TBE). Global Health Press, Singapore. [Google Scholar]
- Evolva. 2020. NootkaShield. https://www.evolva.com/NootKaShield/ (accessed 17 January 2020).
- Falco RC, and Fish D. 1988. Prevalence of Ixodes dammini near the homes of Lyme disease patients in Westchester County, New York. Am. J. Epidemiol 127: 826–830. [DOI] [PubMed] [Google Scholar]
- Fischhoff IR, Bowden SE, Keesing F, and Ostfeld RS. 2019a. Systematic review and meta-analysis of tick-borne disease risk factors in residential yards, neighborhoods, and beyond. BMC Infect. Dis 19: 861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischhoff IR, Keesing F, Pendleton J, DePietro D, Teator M, Duerr STK, Mowry S, Pfister A, LaDeau SL, and Ostfeld RS. 2019b. Assessing effectiveness of recommended residential yard management measures against ticks. J. Med. Entomol 56: 1420–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flor-Weiler LB, Behle RW, and Stafford KC III. 2011. Susceptibility of four tick species, Amblyomma americanum, Dermacentor variabilis, Ixodes scapularis, and Rhipicephalus sanguineus (Acari: Ixodidae), to nootkatone from essential oil of grapefruit. J. Med. Entomol 48: 322–326. [DOI] [PubMed] [Google Scholar]
- de la Fuente J 2018. Controlling ticks and tick-borne diseases—looking forward. Ticks Tick Borne Dis. 9: 1354–1357. [DOI] [PubMed] [Google Scholar]
- Gaitan J, and Millien V. 2016. Stress level, parasite load, and movement pattern in a small-mammal reservoir host for Lyme disease. Can. J. Zool 94: 565–573. [Google Scholar]
- Garnett JM, Connally NP, Stafford KC III, and Cartter ML. 2011. Evaluation of deer targeted interventions on Lyme disease incidence in Connecticut. Publ. Health Rep 126: 446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg HS 2001. Integrated pest management and allocation of control efforts for vector-borne diseases. J. Vector Ecol 26: 32–38. [PubMed] [Google Scholar]
- Ginsberg HS, and Stafford KC III. 2005. Management of ticks and tick-borne diseases, pp. 65–86. In Goodman JL, Dennis DT, and Sonenshine DE (eds.), Tick-borne diseases of humans. ASM Press, Washington, DC. [Google Scholar]
- Ginsberg HS, Bargar TA, Hladik ML, and Lubelczyk C. 2017. Management of arthropod pathogen vectors in North America: minimizing adverse effects on pollinators. J. Med. Entomol 54: 1463–1475. [DOI] [PubMed] [Google Scholar]
- Gomes-Solecki MJ, Brisson DR, and Dattwyler RJ. 2006. Oral vaccine that breaks the transmission cycle of the Lyme disease spirochete can be delivered via bait. Vaccine 24: 4440–4449. [DOI] [PubMed] [Google Scholar]
- Gould LH, Nelson RS, Griffith KS, Hayes EB, Piesman J, Mead PS, and Cartter ML. 2008. Knowledge, attitudes, and behaviors regarding Lyme disease prevention among Connecticut residents, 1999–2004. Vector Borne Zoonotic Dis. 8: 769–776. [DOI] [PubMed] [Google Scholar]
- Graf JF, Gogolewski R, Leach-Bing N, Sabatini GA, Molento MB, Bordin EL, and Arantes GJ. 2004. Tick control: an industry point of view. Parasitology 129(Suppl): S427–S442. [DOI] [PubMed] [Google Scholar]
- Gupta S, Eggers P, Arana A, Kresse B, Rios K, Brown L, Sampson L, and Kploanyi M. 2018. Knowledge and preventive behaviors towards tick-borne diseases in Delaware. Ticks Tick Borne Dis. 9: 615–622. [DOI] [PubMed] [Google Scholar]
- Hahn MB, Jarnevich CS, Monaghan AJ, and Eisen RJ. 2016. Modeling the geographic distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the contiguous United States. J. Med. Entomol 53: 1176–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BF, and Fauci AS. 2009. Malaria control, elimination, and eradication: the role of the evolving biomedical research agenda. J. Infect. Dis 200: 1639–1643. [DOI] [PubMed] [Google Scholar]
- Hayes EB, Maupin GO, Mount GA, and Piesman J. 1999. Assessing the prevention effectiveness of local Lyme disease control. J. Public Health Manag. Pract 5: 84–92. [DOI] [PubMed] [Google Scholar]
- Hinckley AF, Meek JI, Ray JA, Niesobecki SA, Connally NP, Feldman KA, Jones EH, Backenson PB, White JL, Lukacik G, et al. 2016. Effectiveness of residential acaricides to prevent Lyme and other tick-borne diseases in humans. J. Infect. Dis 214: 182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinckley A, Niesobecki S, Hook S, Connally N, Heimer R, and Meek J. 2018. Effectiveness of a rodent-targeted approach to prevent Lyme and other tick-borne diseases in humans. In Abstract, 15th International Conference on Lyme Borreliosis and Other Tick-Borne Diseases, Atlanta, GA, 11–14 September, 2018. [Google Scholar]
- Hook SA, Nelson CA, and Mead PS. 2015. U.S. public’s experience with ticks and tick-borne diseases: results from national healthstyles surveys. Ticks Tick Borne Dis. 6: 483–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James AM, Freier JE, Keirans JE, Durden LA, Mertins JW, and Schlater JL. 2006. Distribution, seasonality, and hosts of the Rocky Mountain wood tick in the United States. J. Med. Entomol 43: 17–24. [PubMed] [Google Scholar]
- Jellison WL 1974. Tularemia in North America, 1930–1974. University of Montana, Missoula, MT. [Google Scholar]
- Johnson J 2009. Dogs, cats, and their people: the place of the family pet and attitudes about pet keeping. M.A. Thesis, University of Waterloo Waterloo, Ontario, Canada. [Google Scholar]
- Jones EH, Hinckley AF, Hook SA, Meek JI, Backenson B, Kugeler KJ, and Feldman KA. 2018. Pet ownership increases human risk of encountering ticks. Zoonoses Public Health 65: 74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan RA, and Egizi A. 2019. The growing importance of lone star ticks in a Lyme disease endemic county: passive tick surveillance in Monmouth County, NJ, 2006–2016. PLoS One 14: e0211778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan RA, and Schulze TL. 2019a. Availability and nature of commercial tick control services in three Lyme disease endemic states. J. Med. Entomol doi: 10.1093/jme/tjz215. [DOI] [PubMed] [Google Scholar]
- Jordan RA, and Schulze TL. 2019b. Ability of two commercially available host-targeted technologies to reduce abundance of Ixodes scapularis (Acari: Ixodidae) in a residential landscape. J. Med. Entomol 56: 1095–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan RA, and Schulze TL. 2020. Artificial accumulation of leaf litter in forest edges on residential properties via leaf blowing is associated with increased numbers of host-seeking Ixodes scapularis (Acari: Ixodidae) nymphs. J. Med. Entomol 57: doi: 10.1093/jme/tjaa033. [DOI] [PubMed] [Google Scholar]
- Jordan RA, Dolan MC, Piesman J, and Schulze TL. 2011. Suppression of host-seeking Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) nymphs after dual applications of plant-derived acaricides in New Jersey. J. Econ. Entomol 104: 659–664. [DOI] [PubMed] [Google Scholar]
- Jordan RA, Schulze TL, and Dolan MC. 2012. Efficacy of plant-derived and synthetic compounds on clothing as repellents against Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae). J. Med. Entomol 49: 101–106. [DOI] [PubMed] [Google Scholar]
- Jordan RA, Schulze TL, Eisen L, and Dolan MC. 2017. Ability of three general use, over-the-counter pesticides to suppress Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) nymphs. J. Am. Mosq. Contr. Assoc 33: 50–55. [DOI] [PubMed] [Google Scholar]
- Keesing F, and Ostfeld RS. 2018. The tick project: testing environmental methods of preventing tick-borne diseases. Trends Parasitol. 34: 447–450. [DOI] [PubMed] [Google Scholar]
- Kilpatrick HJ, LaBonte AM, and Stafford KC. 2014. The relationship between deer density, tick abundance, and human cases of Lyme disease in a residential community. J. Med. Entomol 51: 777–784. [DOI] [PubMed] [Google Scholar]
- Kollars TM Jr,Oliver JH Jr, Masters EJ, Kollars PG, and Durden LA. 2000. Host utilization and seasonal occurrence of Dermacentor species (Acari: Ixodidae) in Missouri, USA. Exp. Appl. Acarol 24: 631–643. [DOI] [PubMed] [Google Scholar]
- Kugeler KJ, Jordan RA, Schulze TL, Griffith KS, and Mead PS. 2016. Will culling white-tailed deer prevent Lyme disease? Zoonoses Public Health 63: 337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RS, Steinlein DB, and Mun J. 2004. Human behaviors elevating exposure to Ixodes pacificus (Acari: Ixodidae) nymphs and their associated bacterial zoonotic agents in a hardwood forest. J. Med. Entomol 41: 239–248. [DOI] [PubMed] [Google Scholar]
- Lehane A, Parise C, Evans C, Beati L, Nicholson WL, and Eisen RJ. 2020. Reported county-level distribution of the American dog tick (Acari: Ixodidae) in the contiguous United States. J. Med. Entomol 57: 131–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac S, da Silva SR, and Sander B. 2019. The economic burden of Lyme disease and the cost-effectiveness of Lyme disease interventions: a scoping review. PLoS One 14: e0210280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maupin GO, Fish D, Zultowsky J, Campos EG, and Piesman J. 1991. Landscape ecology of Lyme disease in a residential area of Westchester County, New York. Am. J. Epidemiol 133: 1105–1113. [DOI] [PubMed] [Google Scholar]
- McKay R, Talbot B, Slatculescu A, Stone A, and Kulkarni MA. 2020. Woodchip borders at the forest ecotone as an environmental control measure to reduce questing tick density along recreational trails in Ottawa, Canada. Ticks Tick Borne Dis. 11: 101361. [DOI] [PubMed] [Google Scholar]
- Mead P, Hook S, Niesobecki S, Ray J, Meek J, Delorey M, Prue C, and Hinckley A. 2018. Risk factors for tick exposure in suburban settings in the Northeastern United States. Ticks Tick Borne Dis. 9: 319–324. [DOI] [PubMed] [Google Scholar]
- Meirelles Richer L, Aroso M, Contente-Cuomo T, Ivanova L, and Gomes-Solecki M. 2011. Reservoir targeted vaccine for Lyme borreliosis induces a yearlong, neutralizing antibody response to OspA in white-footed mice. Clin. Vaccine Immunol 18: 1809–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molaei G, Little EAH, Williams SC, and Stafford KC. 2019. Bracing for the worst—range expansion of the lone star tick in the Northeastern United States. N. Engl. J. Med 381: 2189–2192. [DOI] [PubMed] [Google Scholar]
- Mount GA, and Dunn JE. 1983. Economic thresholds for lone star ticks (Acari: Ixodidae) in recreational areas based on a relationship between CO2 and human subject sampling. J. Econ. Entomol 76: 327–329. [DOI] [PubMed] [Google Scholar]
- Niesobecki S, Hansen A, Rutz H, Mehta S, Feldman K, Meek J, Niccolai L, Hook S, and Hinckley A. 2019. Knowledge, attitudes, and behaviors regarding tick-borne disease prevention in endemic areas. Ticks Tick Borne Dis. 10: 101264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nupp TE, and Swihart RK. 1996. Effect of forest patch area on population attributes of white-footed mice (Peromyscus leucopus) in fragmented landscapes. Can. J. Zool 74: 467–472. [Google Scholar]
- Paddock CD, and Goddard J. 2015. The evolving medical and veterinary importance of the gulf coast tick (Acari: Ixodidae). J. Med. Entomol 52: 230–252. [DOI] [PubMed] [Google Scholar]
- Paddock CD, and Yabsley MJ. 2007. Ecological havoc, the rise of white-tailed deer, and the emergence of Amblyomma americanum-associated zoonoses in the United States. Curr. Top. Microbiol. Immunol 315: 289–324. [DOI] [PubMed] [Google Scholar]
- Paddock CD, Yoshimizu MH, Zambrano ML, Lane RS, Ryan BM, Espinosa A, Hacker JK, Karpathy SE, and Padgett KA. 2018. Rickettsia Species Isolated from Dermacentor occidentalis (Acari: Ixodidae) from California. J. Med. Entomol 55: 1555–1560. [DOI] [PubMed] [Google Scholar]
- Padgett KA, Bonilla D, Eremeeva ME, Glaser C, Lane RS, Porse CC, Castro MB, Messenger S, Espinosa A, Hacker J, et al. 2016. The eco-epidemiology of pacific coast tick fever in California. Plos Negl. Trop. Dis 10: e0005020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panella NA, Karchesy J, Maupin GO, Malan JC, and Piesman J. 1997. Susceptibility of immature Ixodes scapularis (Acari: Ixodidae) to plant-derived acaricides. J. Med. Entomol 34: 340–345. [DOI] [PubMed] [Google Scholar]
- Panella NA, Dolan MC, Karchesy JJ, Xiong Y, Peralta-Cruz J, Khasawneh M, Montenieri JA, and Maupin GO. 2005. Use of novel compounds for pest control: insecticidal and acaricidal activity of essential oil components from heartwood of Alaska yellow cedar. J. Med. Entomol 42: 352–358. [DOI] [PubMed] [Google Scholar]
- Patel M, Lee AD, Clemmons NS, Redd SB, Poser S, Blog D, Zucker JR, Leung J, Link-Gelles R, Pham H, et al. 2019. National update on measles cases and outbreaks—United States, January 1-October 1, 2019. MMWR. Morb. Mortal. Wkly. Rep 68: 893–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paules CI, Marston HD, Bloom ME, and Fauci AS. 2018. Tick-borne diseases—confronting a growing threat. N. Engl. J. Med 379: 701–703. [DOI] [PubMed] [Google Scholar]
- Petersen LR, Beard CB, and Visser SN. 2019. Combatting the increasing threat of vector-borne disease in the United States with a national vector-borne disease prevention and control system. Am. J. Trop. Med. Hyg 100: 242–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister K, and Armstrong R. 2016. Systemically and cutaneously distributed ectoparasiticides: a review of the efficacy against ticks and fleas on dogs. Parasit. Vectors 9: 436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piesman J, and Eisen L. 2008. Prevention of tick-borne diseases. Annu. Rev. Entomol 53: 323–343. [DOI] [PubMed] [Google Scholar]
- Rand PW, Lubelczyk C, Holman MS, Lacombe EH, and Smith RP Jr. 2004. Abundance of Ixodes scapularis (Acari: Ixodidae) after the complete removal of deer from an isolated offshore island, endemic for Lyme Disease. J. Med. Entomol 41: 779–784. [DOI] [PubMed] [Google Scholar]
- Rego ROM, Trentelman JJA, Anguita J, Nijhof AM, Sprong H, Klempa B, Hajdusek O, Tomás-Cortázar J, Azagi T, Strnad M, et al. 2019. Counterattacking the tick bite: towards a rational design of anti-tick vaccines targeting pathogen transmission. Parasit. Vectors 12: 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richer LM, Brisson D, Melo R, Ostfeld RS, Zeidner N, and Gomes-Solecki M. 2014. Reservoir targeted vaccine against Borrelia burgdorferi: a new strategy to prevent Lyme disease transmission. J. Infect. Dis 209: 1972–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochlin I, Ninivaggi DV, and Benach JL. 2019. Malaria and Lyme disease—the largest vector-borne US epidemics in the last 100 years: success and failure of public health. BMC Publ. Health 19: 804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R, Lindsey NP, Fischer M, Gregory CJ, Hinckley AF, Mead PS, Paz-Bailey G, Waterman SH, Drexler NA, Kersh GJ, et al. 2018. Vital signs: trends in reported vectorborne disease cases—United States and Territories, 2004–2016. Morb. Mortal. Wkly. Rep 67: 496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi C, Stromdahl EY, Rohrbeck P, Olsen C, and DeFraites RF. 2015. Characterizing the relationship between tick bites and Lyme disease in active component U.S. Armed Forces in the eastern United States. MSMR. 22: 2–10. [PubMed] [Google Scholar]
- Schuijt TJ, Hovius JW, van der Poll T, van Dam AP, and Fikrig E. 2011. Lyme borreliosis vaccination: the facts, the challenge, the future. Trends Parasitol. 27: 40–47. [DOI] [PubMed] [Google Scholar]
- Schulze TL, and Jordan RA. 2019. Early season applications of bifenthrin suppress Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) nymphs. J. Med. Entomol doi: 10.1093/jme/tjz202. [DOI] [PubMed] [Google Scholar]
- Schulze TL, Jordan RA, and Hung RW. 1997. Availability and nature of commercial tick control services in established and emerging Lyme disease areas of New Jersey. J. Spirochetal Tick-borne Dis 4: 44–48. [Google Scholar]
- Schulze TL, Jordan RA, Schulze CJ, Healy SP, Jahn MB, and Piesman J. 2007. Integrated use of 4-Poster passive topical treatment devices for deer, targeted acaricide applications, and Maxforce TMS bait boxes to rapidly suppress populations of Ixodes scapularis (Acari: Ixodidae) in a residential landscape. J. Med. Entomol 44: 830–839. [DOI] [PubMed] [Google Scholar]
- Schulze L, Jordan RA, Dolan MC, Dietrich G, Healy SP, and Piesman J. 2008. Ability of 4-poster passive topical treatment devices for deer to sustain low population levels of Ixodes scapularis (Acari: Ixodidae) after integrated tick management in a residential landscape. J. Med. Entomol 45: 899–904. [DOI] [PubMed] [Google Scholar]
- Schulze TL, Jordan RA, Williams M, and Dolan MC. 2017. Evaluation of the select tick control system (TCS), a host-targeted bait box, to reduce exposure to Ixodes scapularis (Acari: Ixodidae) in a Lyme disease endemic area of New Jersey. J. Med. Entomol 54: 1019–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen AK, Mead PS, and Beard CB. 2011. The Lyme disease vaccine–a public health perspective. Clin. Infect. Dis 52(Suppl 3): s247–s252. [DOI] [PubMed] [Google Scholar]
- Sonenshine D 2018. Range expansion of tick disease vectors in North America: implications for spread of tick-borne disease. Int. J. Environ. Res. Publ. Health 15: 478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshine DE, and Haines G. 1985. A convenient method for controlling populations of the American dog tick, Dermacentor variabilis (Acari: Ixodidae), in the natural environment. J. Med. Entomol 22: 577–583. [DOI] [PubMed] [Google Scholar]
- Spielman A 1994. The emergence of Lyme disease and human babesiosis in a changing environment. Ann. N. Y. Acad. Sci 740: 146–156. [DOI] [PubMed] [Google Scholar]
- Spielman A, Wilson ML, Levine JF, and Piesman J. 1985. Ecology of Ixodes dammini-borne human babesiosis and Lyme disease. Annu. Rev. Entomol 30: 439–460. [DOI] [PubMed] [Google Scholar]
- Springer YP, Eisen L, Beati L, James AM, and Eisen RJ. 2014. Spatial distribution of counties in the continental United States with records of occurrence of Amblyomma americanum (Ixodida: Ixodidae). J. Med. Entomol 51: 342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford KC III. 1997. Pesticide use by licensed applicators for the control of Ixodes scapularis (Acari: Ixodidae) in Connecticut. J. Med. Entomol 34: 552–558. [DOI] [PubMed] [Google Scholar]
- Stafford KC III. 2007. Tick management handbook: an integrated guide for homeowners, pest control operators, and public health officials for the prevention of tick-associated disease, 2nd ed. The Connecticut Agricultural Experiment Station, Bull. No. 1010, New Haven, CT. [Google Scholar]
- Stafford KC III. 2017. Prevention of ticks and tick-associated disease in companion animals. Connecticut Agricultural Experiment Station Fact Sheet, New Haven, CT, 9 pp., https://portal.ct.gov/CAES/Publications [Google Scholar]
- Stafford KC III, and Magnarelli LA. 1993. Spatial and temporal patterns of Ixodes scapularis (Acari: Ixodidae) in southeastern Connecticut. J. Med. Entomol 30: 762–771. [DOI] [PubMed] [Google Scholar]
- Stafford KC III, and Williams SC. 2017. Deer-targeted methods: a review of the use of topical acaricides for the control of ticks on white-tailed deer. J. Integr. Pest Manage 8: 19. [Google Scholar]
- Stafford KC III, Williams SC, and Molaei G. 2017. Integrated pest management in controlling ticks and tick-associated diseases. J. Integr. Pest Manage 8: 28. [Google Scholar]
- Stafford KC III, Molaei G, Little EAH, Paddock CD, Karpathy SE, and Labonte AM. 2018. Distribution and establishment of the lone star tick in Connecticut and implications for range expansion and public health. J. Med. Entomol 55: 1561–1568. [DOI] [PubMed] [Google Scholar]
- Stafford KC III, Williams SC, van Oosterwijk JG, Linske MA, Zatechka S, Richer LM, Molaei G, Przybyszewski C, and Wikel SK. 2020. Field evaluation of a novel oral reservoir-targeted vaccine against Borrelia burgdorferi utilizing an inactivated whole-cell bacterial antigen expression vehicle. Exp. Appl. Acarol 80: 257–268. [DOI] [PubMed] [Google Scholar]
- Steere AC, and Livey I. 2012. Lyme disease vaccines, pp. 1122–1132. In Plotkin SA, Orenstein WA, and Offit PA (eds.), Vaccines, 6th ed. Elsevier, Philadelphia, PA. [Google Scholar]
- Stewart KM, Bowyer T, and Weisberg PJ. 2011. Spatial use of landscapes, pp. 181–217. In Hewitt DG (ed.), Biology and management of white-tailed deer. CRC Press, New York, NY. [Google Scholar]
- Süss J 2008. Tick-borne encephalitis in Europe and beyond—The epidemiological situation as of 2007. Eurosurveillance 13: 717–727. [PubMed] [Google Scholar]
- Telford SR III. 2018. Deer reduction is a cornerstone of integrated deer tick management. J. Integr. Pest Manage 8: 25. [Google Scholar]
- Telford SR III, and Goethert HK. 2020. Ecology of Francisella tularensis. Annu. Rev. Entomol 65: 351–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Biologic. 2020. US BIOLOGIC delivers disease prevention by providing orally delivered vaccines to address the world’s great health challenges. https://usbiologic.com/ (accessed 17 January 2020).
- U.S. Bureau of Labor Statistics. 2017. American time use survey. https://www.bls.gov/tus/charts.htm (accessed 23 March 2020).
- USDA (United States Department of Agriculture). 2018. A national roadmap for integrated pest management. The Federal IPM Coordinating Committee. https://www.ars.usda.gov/ARSUserFiles/OPMP/IPM%20Road%20Map%20FINAL.pdf (accessed 10 January 2020).
- Vázquez M, Muehlenbein C, Cartter M, Hayes EB, Ertel S, and Shapiro ED. 2008. Effectiveness of personal protective measures to prevent Lyme disease. Emerg. Infect. Dis 14: 210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White A, and Gaff H. 2018. Application of tick control technologies for blacklegged, lone star, and American dog ticks. J. Integr. Pest Manage 9: 12. [Google Scholar]
- Williams SC, Linske MA, and Ward JS. 2017. Long-term effects of Berberis thunbergii (Ranunculales: Berberidaceae) management on Ixodes scapularis (Acari: Ixodidae) abundance and Borrelia burgdorferi (Spirochaetales: Spirochaetaceae) prevalence in Connecticut, USA. Environ. Entomol 46: 1329–1338. [DOI] [PubMed] [Google Scholar]
- Williams SC, Little EAH, Stafford KC III, Molaei G, and Linske MA. 2018a. Integrated control of juvenile Ixodes scapularis parasitizing Peromyscus leucopus in residential settings in Connecticut, United States. Ticks Tick Borne Dis. 9: 1310–1316. [DOI] [PubMed] [Google Scholar]
- Williams SC, Stafford KC III, Molaei G, and Linske MA. 2018b. Integrated control of nymphal Ixodes scapularis: effectiveness of white-tailed deer reduction, the entomopathogenic fungus Metarhizium anisopliae, and fipronil-based rodent bait boxes. Vector Borne Zoonotic Dis. 18: 55–64. [DOI] [PubMed] [Google Scholar]
- Williams SC, van Oosterwijk JG, Linske MA. Zatechka S, Richer LM, Przybyszewski C, Wikel SK, and Stafford KC III. 2020. Administration of an orally-delivered substrate targeting a mammalian zoonotic pathogen reservoir population: novel application and biomarker analysis. Vector Borne Zoonotic Dis. 20. doi: 10.1089/vbz.2019.2612. [DOI] [PubMed] [Google Scholar]
- Wolff JO 1985. The effects of density, food, and interspecific interference on home range size in Peromyscus leucopus and Peromyscus maniculatus. Can. J. Zool 63: 2657–2662. [Google Scholar]
- Xu G, Mather TN, Hollingsworth CS, and Rich SM. 2016. Passive surveillance of Ixodes scapularis (Say), their biting activity, and associated pathogens in Massachusetts. Vector Borne Zoonotic Dis. 16: 520–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman RH, McWherter GR, and Bloemer SR. 1987. Role of small mammals in population dynamics and dissemination of Amblyomma americanum and Dermacentor variabilis (Acari: Ixodidae) at Land Between The Lakes, Tennessee. J. Med. Entomol 24: 370–375. [DOI] [PubMed] [Google Scholar]


