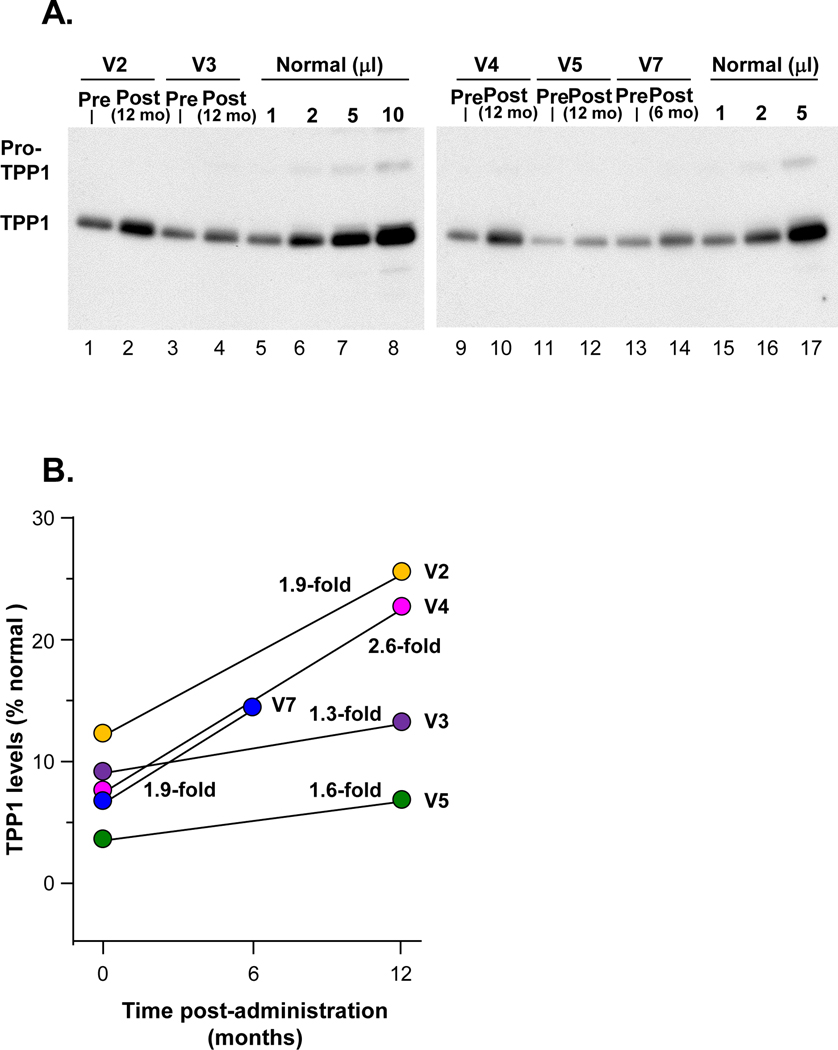
Figure 3. Human TPP1 in CSF following AAVrh.10hCLN2 administration to cohort 1.
CSF was analyzed from TPP1 by Western analysis. The IRB approved protocol allowed for CSF sampling before therapy and only 1 time after therapy. A. Western analysis. Lanes 1–2, participant V2; lanes 3–4, participant V3; lanes 5–8, 1, 2, 5 and 10 μl, respectively, of combined CSFs of three healthy children (1:1:1 volume mix) as a positive control. Lanes 9–10, participant V4; lanes 11–12, participant V5; lanes 13–14, participant V7; lanes 15–17, 1, 2 and 5 ml, respectively, of combined CSFs of three healthy subjects (1:1:1 volume mix) as a positive control. B. Quantitation of TPP1 in CSF before and after therapy expressed as percent normal TPP1 in CSF following AAVrh.10hCLN2 administration compared to pre-administration (pre vs post % normal, p<0.03, paired two-tailed t-test). V7 received the lower dose (2.85×1011 gc).