Abstract
Previous studies suggested that the SARS-CoV-2 virus may gain access to the brain by using a route along the olfactory nerve. However, there is a general consensus that the obligatory virus entry receptor, angiotensin converting enzyme 2 (ACE2), is not expressed in olfactory receptor neurons, and the timing of arrival of the virus in brain targets is inconsistent with a neuronal transfer along olfactory projections. We determined whether nervus terminalis neurons and their peripheral and central projections may provide an alternative route from the nose to the brain. Nervus terminalis neurons were double-labeled with antibodies against ACE2 and nervus terminalis markers in postnatal mice. We show that most nervus terminalis neurons with cell bodies in the region between the olfactory epithelium and the olfactory bulb express ACE2, and therefore may provide a direct route for the virus from the nasal epithelium and Bowman’s glands to brain targets, including the telencephalon and diencephalon.
Keywords: Nervus terminalis, ACE2, SARS-CoV-2, COVID-19, brain infection, olfactory system
INTRODUCTION
Many previous reports have suggested that the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) gains access to the brain by using an olfactory route from the nose to the brain (Bougakov et al., 2020; Briguglio et al., 2020; Butowt and Bilinska, 2020; Li et al., 2020; Meinhardt et al., 2020; Natoli et al., 2020; Zubair et al., 2021; Burks et al., 2021), similar to other neuro-invasive viruses that have been shown to infect olfactory receptor neurons and spread from these first-order olfactory neurons to secondary and tertiary targets in the brain (Barnett and Perlman, 1993; van Riel et al., 2015; Dubé et al., 2018). Indeed, it has been shown that SARS-CoV-2 can accumulate in various brain regions, in animal models (reviewed in: Butowt and von Bartheld, 2020; Rathnasinghe et al., 2020) and in a small number of human patients with COVID-19 (Ellul et al., 2020; Matschke et al., 2020; Meinhardt et al., 2020; Mukerji and Solomon, 2020; Solomon, 2021).
However, the route along the olfactory nerve from the nose to the brain is controversial for SARS-CoV-2, primarily for two reasons: (1) the olfactory receptor neurons do not express the obligatory virus entry receptor, the angiotensin-converting enzyme 2 (ACE2), or expression is restricted to a very small subset of these neurons (Butowt and von Bartheld, 2020; Cooper et al., 2020). Because sustentacular cells tightly enwrap olfactory receptor neurons (Liang, 2020), these ACE2-expressing support cells can easily be mistaken for olfactory receptor neurons, resulting in false positive identification. (2) The timeline of appearance of SARS-CoV-2 in the brain is inconsistent with a “neuron-hopping” mode: infection of third-order olfactory targets should occur with a significant delay after infection of the olfactory epithelium, as has been reported for other neuro-invasive viruses (Barnett et al., 1995), but instead the hypothalamus and brainstem are reported to be infected as early as, or even earlier than, the olfactory bulb (de Melo et al., 2020; Zheng et al., 2020), and SARS-CoV-2 may even skip the olfactory nerve and olfactory bulb on its way to brain infection (Winkler et al., 2020; Zhou et al., 2020; Carossino et al., 2021). These findings have raised doubt about the notion that the olfactory nerve serves as a major conduit for brain infection in COVID-19.
With few exceptions (Briguglio et al., 2020; Butowt and von Bartheld, 2020), studies suggesting an olfactory route for SARS-CoV-2 to achieve brain infection fail to consider an alternative route from the nose to the brain, the route via the nervus terminalis. Many peripheral processes of the nervus terminalis innervate the olfactory epithelium, the blood vessels below this epithelium, as well as cells in Bowman’s glands (Larsell, 1950), and the central processes of some of these neurons extend to various targets in the forebrain as far caudal as the hypothalamus (Pearson, 1941; Larsell, 1950; Schwanzel-Fukuda et al., 1987; Demski, 1993; von Bartheld, 2004). Some of the nervus terminalis neurons are in direct contact with spaces containing cerebrospinal fluid (CSF) in the region of the olfactory nerve and bulb (Jennes, 1987). About 30–40% of the neurons of the nervus terminalis express gonadotropin-releasing hormone (GnRH) and some of these neurons may release GnRH into blood vessels below the olfactory epithelium (Jennes, 1987; Schwanzel-Fukuda et al., 1987), while other neuronal populations of the nervus terminalis system are thought to regulate blood flow and blood pressure in the nose and forebrain (Larsell, 1918; Oelschläger et al., 1987; Ridgway et al., 1987). These properties make the nervus terminalis a strong candidate for expression of ACE2, known to regulate blood flow and blood pressure in many tissues (Tikellis and Thomas, 2012). Expression of ACE2 in the nervus terminalis would suggest that this cranial nerve is a plausible alternative to the olfactory nerve for SARS-CoV-2 virus to gain access to the brain. However, it has not been previously examined and reported whether nervus terminalis neurons express the obligatory viral entry receptor, ACE2. We have therefore examined whether ACE2 is expressed in these neurons in an animal model, the postnatal mouse.
MATERIALS AND METHODS
Animals and tissue processing
A total of six wildtype C57BL/6J mice (Jackson Laboratory) at age 3–4 weeks old were used to obtain tissue material for experiments. Mice were housed with a 12/12 h light/dark cycle and given access to water and food ad libitum. All animal experiments were approved by the local ethics committee for animal research at Bydgoszcz (Poland). Immediately after cervical dislocation, the mice were exsanguinated and tissues were dissected. Olfactory epithelium and brain were frozen at −80 °C for storage and further usage or fixed 3 h at 4 °C in 4% (w/v) freshly prepared paraformaldehyde in phosphate-buffered saline (PBS, pH 7,5) and then incubated in 25% (w/v) sucrose/PBS at 4° C for 16–24 h, frozen in Tissue-Tek O.C.T. (Sakura Finetek), and cryosectioned at 10–12 μm using a Leica CM1850 cryostat.
ACE2 −/− knockout (ACE2 KO) control
To verify the specificity of the ACE2 antibody, an ACE2 knock-out (KO) mouse line was obtained from Taconic (strain #18180). Two male homozygous ACE2 KO mice at age 3 weeks old were processed and immunolabeled as described below for wildtype mice. Genotyping was performed according to the manufacturer’s suggested PCR protocol. Lack of an ACE2 protein band was confirmed by using Western blots (not shown).
Immunocytochemistry and co-localization analysis
For single immunofluorescence labeling, frozen sections cut at 10–12 μm were stained overnight with a mixture of primary goat anti-ACE2 at 1/500 dilution and rabbit anti-GnRH (gonadotropin releasing hormone) at 4 °C. Next day sections were washed five times in PBST (PBS with 0.05% Triton X-100) and incubated with a mixture of secondary anti-rabbit-AF488 antibody and anti-goat-AF594 at 1/500 dilution for 60 min at room temperature. Next, sections were stained for 5 min at room temperature in Hoechst 33258 (Sigma-Aldrich) to visualize cell nuclei and embedded in antifade medium (Vector laboratories). Alternatively, cryosections were stained with rabbit polyclonal anti-CHAT (choline acetyltransferase) instead of rabbit anti-GnRH antibody in the double staining primary antibody mixture. Occasionally, sections were stained with anti-OMP (olfactory marker protein) at 1/500 dilution in PBST, following the same protocol. After immunocytochemical reactions, sections were analyzed on a Nikon Eclipse 80i microscope and images were taken using a Nikon DP80 camera. Microscopic images were processed using CellSense software (Nikon corp). Antibodies used in this study are listed in Supplemental Material, Table S1.
Cell counting and statistical analysis
For counting double labeled cells, four male wildtype mice at age 3–4 weeks old were used. Approximately every third coronal cryosection (10–12 μm thickness) was stained as described above, and positive cells were counted in tissue sections under a fluorescent microscope as indicated in Fig. 1 (the medial region from the posterior olfactory epithelium to the caudal end of the olfactory bulb). For each animal, the percentage of double labeled GnRH+/ACE2+ cells was calculated in relation to the total number of GnRH-positive cells detected. The same protocol was applied for counting cholinergic nervus terminalis neurons co-labeled with ACE2. A total number of approximately 100 GnRH-positive neurons and 50 CHAT-positive neurons were counted from four animals. The results were analyzed using GraphPad Prism software. Results are presented as mean ± SEM.
Fig. 1.
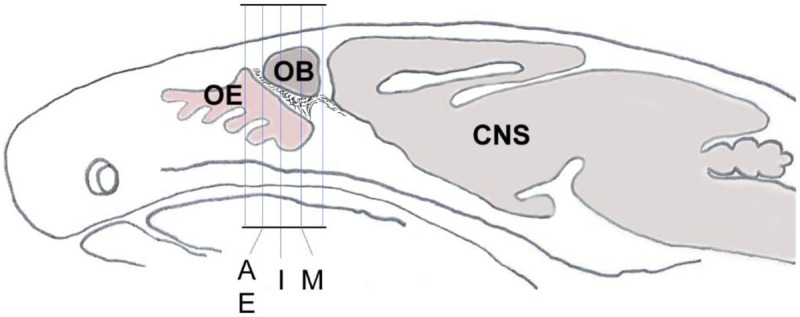
Schematic sagittal section through a mouse head shows the orientation and planes of tissue sections from Fig. 2A, E, I and M that were used for demonstration of double-immunolabeling and cell counting.
RESULTS
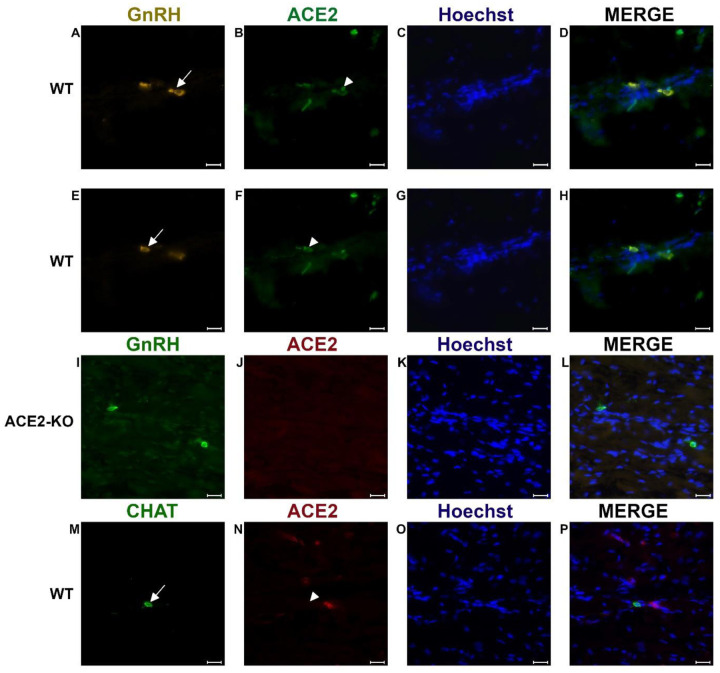
It was previously shown that GnRH is a marker for a major fraction of nervus terminalis neurons (Jennes, 1987; Schwanzel-Fukuda et al., 1987; Demski, 1993; Kim et al., 1999; von Bartheld, 2004). Immunolabeling for GnRH in 3–4 week-old mice showed labeled cells localized along the olfactory nerve between the olfactory epithelium and the olfactory bulbs (Fig. 2A–H), as expected from previous studies in rodents (Schwanzel-Fukuda et al., 1986; Wirsig and Leonard, 1986; Schwanzel-Fukuda et al., 1987). The majority of the GnRH-positive nervus terminalis neurons was located along the midline in the posterior part of the olfactory epithelium and adjacent to the olfactory bulbs. Preliminary examination revealed that these cells were in the same vicinity as cells labeled with the ACE2 antibody (Fig. 2A–B, E–F). The large majority of GnRH-positive nervus terminalis neurons were fusiform and unipolar in shape.
Fig. 2.
Examples of double immunofluorescent labeling for nervus terminalis neuronal markers GnRH (A, E) or CHAT (M) and ACE2 (B, F, J, N) in the medial region adjacent to the olfactory bulbs as indicated in Fig. 1. Panels A-D and E-H show slightly different focal planes to demonstrate the morphology of the two or three different neurons. Nuclei are stained with Hoechst 33258 (C, G, K, O). Merged images are shown in the last column (D, H, L, P). The neuronal somas labeled with GnRH (A, E) are co-labeled with ACE2 (B, F) as shown after merging (D, H). GnRH positive cells in the ACE2 knock-out mouse (I) are not labeled with ACE2 (J). The majority of cholinergic neurons are not labeled with ACE2 (M, N), as quantified in Fig. 3. Control sections probed without primary antibodies or with control rabbit IgG had no detectable signal (not shown). Arrows and triangles indicate double-labeled neurons or lack thereof. Scale bars: 20 μm.
Double-label of GnRH and ACE2 neurons with quantification
In order to determine whether some neurons of the nervus terminalis contained both GnRH and ACE2, and to estimate the number of such cells, we performed double immunolabeling experiments, and single- and double-labeled cells were counted on 15–20 sections from three different animals. The analyzed olfactory epithelium and olfactory bulb region and section’s cutting plane are as indicated in Fig. 1. After counting a total of 107 double positive (GnRH+/ACE2+) cells it was calculated that 90.9% of them were double-labeled (Fig. 3). Controls included omission of the primary antibody (not shown) and double immunofluorescent reactions performed using cryosections derived from an ACE2 knockout mouse as shown in Fig. 2 (I–L). The GnRH-positive cells were never positive for olfactory marker protein (OMP), a marker for mature olfactory receptor neurons (Fig. S1, supplementary materials). The total number of GnRH+ nervus terminalis neurons per mouse was estimated to be approximately 125, which is very similar to a previous serial section analysis in hamster (about 130–140 GnRH+ neurons, Wirsig and Leonard, 1986).
Fig. 3.
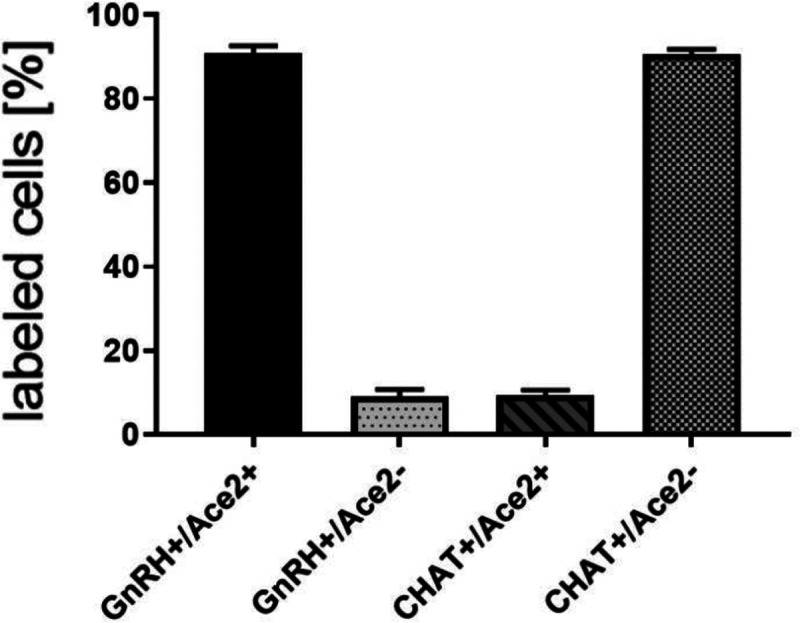
Quantification of co-localization of GnRH and ACE2, and CHAT and ACE2 in nervus terminalis neurons. The large majority of GnRH-positive neurons is also ACE2-positive. In contrast, the majority of CHAT-positive (cholinergic) nervus terminalis neurons lack ACE2-expression. The total number of counted GnRH-positive or CHAT-positive neurons was set at 100%. Error bars represent ± SEM.
Double-label of CHAT and ACE2 neurons with quantification
The nervus terminalis complex is comprised of several distinct heterogenic populations of neurons. In addition to GnRH cells which form the majority of nervus terminalis cells, the second largest nervus terminalis subpopulation are cholinergic neurons that can be identified by the presence of choline acetyltransferase (CHAT) or cholinesterase (Wirsig and Leonard, 1986; Demski, 1993; von Bartheld, 2004). Therefore, CHAT neurons were also double labeled with ACE2 and the fraction of CHAT-positive and ACE2-positive neurons was estimated out of a total of 51 CHAT-positive neurons in four animals. In contrast to GnRH+/ACE2+ cells, only a minor fraction of about 9.4% of CHAT-positive cells were labeled with ACE2 which suggests that very few cholinergic nervus terminalis cells express ACE2 protein (Fig. 3). The large majority of CHAT-positive and also ACE2-positive nervus terminalis neurons were fusiform and unipolar in shape. Control experiments included omission of primary antibody (not shown) and double immunofluorescent reactions performed using cryosections derived from ACE2 knockout mouse (see below). The total number of CHAT-positive nervus terminalis neurons per mouse was estimated to be approximately 60–70. This is less than the 130–140 acetylcholinesterase containing nervus terminalis neurons in hamster (Wirsig and Leonard, 1986), but it is known that only a fraction of neurons containing acetylcholinesterase actually are cholinergic (Schwanzel-Fukuda et al., 1986).
Knock-out control mice for ACE2 −/−
Immunolabeling experiments did not reveal any signal beyond background when the primary antibodies were omitted. For more precise visualization of background ACE2 staining, tissue derived from ACE2 knock-out mouse was used. Experiments using Western blotting technique showed lack of an ACE2-specific band in total protein extract obtained from ACE2 −/− animals (results not shown). Therefore, these sections were also used for double immunolabeling experiments with ACE2 antibody and results showed that, as expected, GnRH-positive cells were negative for ACE2 in tissue sections from the knock-out animals (Fig. 2 I–L).
DISCUSSION
Our experiments confirmed the locations and approximate numbers of GnRH-positive neurons of the nervus terminalis in rodents (Schwanzel-Fukuda et al., 1986; Wirsig and Leonard, 1986; Schwanzel-Fukuda et al., 1987). In mouse, we found that the number of CHAT-positive neurons was about half of the number of GnRH-positive neurons. Interestingly, the large majority of GnRH-positive neurons also expressed ACE2, while only a small fraction of CHAT-positive neurons co-localized ACE2. Previous studies have suggested that the CHAT-positive neurons more often were multipolar and possibly associated with an autonomic function, such as innervating Bowman glands, while GnRH-positive neurons were thought to be sensory and/or may have neurosecretory functions (Wirsig and Leonard, 1986; Schwanzel-Fukuda et al., 1986).
Mice have been most often used as model systems for ACE2 expression, for localization of SARS-CoV-2 in the olfactory epithelium, and to study neuro-invasion of the brain along the olfactory route (Butowt and von Bartheld, 2020; Cooper et al., 2020; Rathnasinghe et al., 2020). Mice have the advantage that a large number of mutants are available (Butowt and von Bartheld, 2020), but they normally express an ACE2 version that binds SARS-CoV-2 with low affinity (Damas et al., 2020). Therefore, to study SARS-CoV-2 infection in mice, a mouse-adapted virus has to be used (Leist et al., 2020), or mice have to be engineered to express human ACE2.
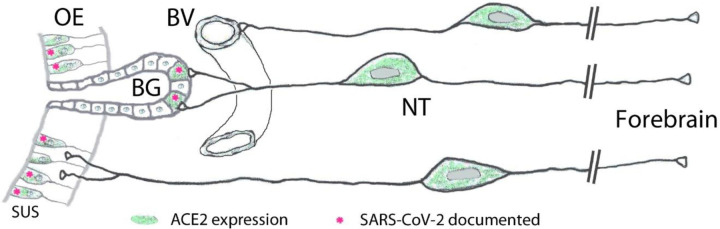
Importantly, our finding of ACE2 expression in the large majority of GnRH-expressing nervus terminalis neurons suggests that this cranial nerve is a more plausible conduit for brain infection than the olfactory neurons that entirely or for the most part lack ACE2 expression (Butowt and von Bartheld, 2020; Cooper et al., 2020). The nervus terminalis neurons may obtain the SARS-CoV-2 directly from infected cells in Bowman’s glands, or through free nerve endings within the olfactory epithelium, many parts of which degenerate when sustentacular cells are infected by SARS-CoV-2 (Bryche et al., 2020). As illustrated in Fig. 4, the nervus terminalis thus has multiple venues to bind the virus, while the lack of ACE2 in olfactory receptor neurons appears to be a barrier to virus transfer.
Fig. 4.
Peripheral projections of nervus terminalis (NT) neurons and their presumptive relationship with ACE2-expressing neurons in the olfactory epithelium and known SARS-CoV-2 infection. NT neurons innervate blood vessels (BV), Bowman gland (BG) cells, and the olfactory epithelium (OE). Peripheral projections of NT neurons according to Larsell (1950). Cells expressing ACE2 are indicated in green, including sustentacular cells (SUS) and BG cells. Both of these cell types have been shown to express ACE2 (Bilinska et al., 2020; Brann et al., 2020; Chen et al., 2020; Ye et al., 2020; Zhang et al., 2020; Klingenstein et al., 2021). Cell types that have been documented to be infected by SARS-CoV-2 are indicated with pink asterisks. SARS-CoV-2 localization in SUS cells according to Bryche et al., 2020; de Melo et al., 2020; Leist et al., 2020; Ye et al., 2020; Zhang et al., 2020; Zheng et al., 2020, and in BG cells according to Bryche et al., 2020; Leist et al., 2020; Ye et al., 2020.
Another important aspect is that the timeline of appearance of SARS-CoV-2 in the brain fits the nervus terminalis projections, with an explosive appearance of the virus in the forebrain in some mouse models (Winkler et al., 2020; Zheng et al., 2020; Zhou et al., 2020; Carossino et al., 2021), rather than a gradual transfer along the olfactory projections as would be expected from a virus that gains access to the brain via olfactory projections (Barnett and Perlman, 1993). The nervus terminalis has direct projections into the forebrain, reaching as far caudal as the hypothalamus (von Bartheld, 2004), and this could explain why the virus reaches the brain and cerebrospinal fluid (CSF) spaces much faster than seems possible via “neuron hopping” along olfactory projections. Most of the virus-containing axons in the olfactory nerve demonstrated by Melo et al. (2020) do not express olfactory marker protein, suggesting that they are not axons belonging to olfactory receptor neurons, and therefore may be nervus terminalis axons which also project through the olfactory nerve (Larsell, 1950).
On a comparative note, since dolphins and whales have a much larger number of nervus terminalis cells than any other vertebrates (Oelschläger et al., 1987), and these marine mammals express ACE2 that is highly susceptible to SARS-CoV-2 infection (Damas et al., 2020), our finding of ACE2 in nervus terminalis cells suggests that these animals may be more vulnerable to brain infection via the nervus terminalis – even in the absence of an olfactory system.
In humans, the number of nervus terminalis neurons is relatively small (a few hundred to a few thousand neurons depending on age, Brookover, 1917; Larsell, 1950; Jin et al., 2019). However, such a relatively small number may be sufficient to mediate viral infection, especially considering that the nervus terminalis directly innervates secretory cells of the Bowman’s glands (Larsell, 1950) that are known to express ACE2 (Brann et al., 2020; Chen et al., 2020; Cooper et al., 2020; Ye et al., 2020; Zhang et al., 2020; Klingenstein et al., 2021) and readily become infected with SARS-CoV-2 (Ye et al., 2020; Leist et al., 2020; Meinhardt et al., 2020; Zhang et al., 2020; Zheng et al., 2020) (Fig. 4). In addition, the nervus terminalis has many free nerve endings within the olfactory epithelium (Larsell, 1950) – an epithelium that is heavily damaged when ACE2-expressing sustentacular cells become infected and degenerate (Bryche et al., 2020). Finally, a major component of the nervus terminalis innervates blood vessels below the olfactory epithelium and projects via cerebrospinal fluid (CSF)-containing spaces (Larsell, 1950; Jennes, 1987). Some nervus terminalis neurons have direct projections to the hypothalamus (Pearson, 1941; Larsell, 1950; von Bartheld, 2004), a brain region that may serve as a hub for virus spread throughout the brain (Nampoothiri et al., 2020; Zheng et al., 2020).
Another argument to consider the nervus terminalis as an alternative to the olfactory route is that neuro-invasion in most animal models is highly variable, even in the same species and transgenic model (Jiang et al., 2020; Oladunni et al., 2020; Rathnasinghe et al., 2020; Winkler et al., 2020; Ye et al., 2020; Zheng et al., 2020; Zhou et al., 2020), and this is despite a very consistent olfactory system in terms of numbers of neurons, gene expression and projections. The nervus terminalis, on the other hand, is known for its unusually large variability between individuals of the same species or even when comparing the right side with the left side of the same individual (Larsell, 1918; Jin et al., 2019). Such numerical differences can approach or even exceed an entire order of magnitude (Schwanzel-Fukuda et al., 1987; Jin et al., 2019) – and thus may explain the reported large variability in neuro-invasion along this route. Taken together, nervus terminalis neurons, for the above reasons, should be considered as a plausible alternative to the olfactory projections for neuro-invasion of SARS-CoV-2 from the nose to the brain in COVID-19.
Supplementary Material
ACKNOWLEDGMENTS
Supported by the “Excellence Initiative-Research University” programme at the Nicolaus Copernicus University (R.B.), and grant GM103554 from the National Institutes of Health (C.S.v.B.).
REFERENCES
- Barnett E.M., and Perlman S. (1993). The olfactory nerve and not the trigeminal nerve is the major site of CNS entry for mouse hepatitis virus, strain JHM. Virology. 194(1), 185–191. [DOI] [PubMed] [Google Scholar]
- Barnett E.M., Evans G.D., Sun N., Perlman S., and Cassell M.D. (1995). Anterograde tracing of trigeminal afferent pathways from the murine tooth pulp to cortex using herpes simplex virus type 1. J Neurosci. (4), 2972–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilinska K., Jakubowska P., Von Bartheld C.S., and Butowt R. (2020). Expression of the SARS-CoV-2 Entry Proteins, ACE2 and TMPRSS2, in Cells of the Olfactory Epithelium: Identification of Cell Types and Trends with Age. ACS Chem Neurosci. 11(11), 1555–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougakov D., Podell K., and Goldberg E. (2020). Multiple Neuroinvasive Pathways in COVID-19. Mol Neurobiol. 29,1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briguglio M., Bona A., Porta M., Dell’Osso B., Pregliasco F.E., and Banfi G. (2020). Disentangling the hypothesis of host dysosmia and SARS-CoV-2: The bait symptom that hides neglected neurophysiological routes. Front Physiol. 11, 671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann D.H., Tsukahara T., Weinreb C., Lipovsek M., Van den Berge K., Gong B., et al. (2020). Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci Adv. 6(31):eabc5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookover C. (1917). The peripheral distribution of the nervus terminalis in an infant. J Comp Neurol. 28, 349–360. [Google Scholar]
- Bryche B., St Albin A., Murri S., Lacôte S., Pulido C., Ar Gouilh M., et al. (2020). Massive transient damage of the olfactory epithelium associated with infection of sustentacular cells by SARS-CoV-2 in golden Syrian hamsters. Brain Behav Immun. 89, 579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burks S.M., Rosas-Hernandez H., Alenjandro Ramirez-Lee M., Cuevas E., and Talpos J.C. (2021). Can SARS-CoV-2 infect the central nervous system via the olfactory bulb or the blood-brain barrier? Brain Behav Immun. 20, 32489–32492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butowt R., and Bilinska K. (2020). SARS-CoV-2: Olfaction, Brain Infection, and the Urgent Need for Clinical Samples Allowing Earlier Virus Detection. ACS Chem Neurosci. 11(9),1200–1203. [DOI] [PubMed] [Google Scholar]
- Butowt R., and von Bartheld C.S. (2020). Anosmia in COVID-19: Underlying Mechanisms and Assessment of an Olfactory Route to Brain Infection. Neuroscientist. doi: 10.1177/1073858420956905. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carossino M., Montanaro P., O’Connell A., Kenney D., Gertje H., Grosz K.A., et al. (2021). Fatal neuroinvasion of SARS-CoV-2 in K18-hACE2 mice is partially dependent on hACE2 expression. bioRxiv [Preprint] doi: 10.1101/2021.01.13.425144. (accessed on February 26, 2021). [DOI] [Google Scholar]
- Chen M., Shen W., Rowan N. R., Kulaga H., Hillel A., Ramanathan M. Jr, et al. (2020). Elevated ACE-2 expression in the olfactory neuroepithelium: implications for anosmia and upper respiratory SARS-CoV-2 entry and replication. Eur Resp J. 56(3), 2001948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper K.W., Brann D.H., Farruggia M.C., Bhutani S., Pellegrino R., Tsukahara T., et al. (2020). COVID-19 and the Chemical Senses: Supporting Players Take Center Stage. Neuron. 107(2), 219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damas J., Hughes G.M., Keough K.C., Painter C.A., Persky N.S., Corbo M., et al. (2020). Broad host range of SARS-CoV-2 predicted by comparative and structural analysis of ACE2 in vertebrates. Proc Natl Acad Sci U S A. 117(36), 22311–22322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Melo G.D., Lazarini F., Levallois S., Hautefort C., Michel V., Larrous F., et al. (2020). COVID-19 associated olfactory dysfunction reveals SARS-CoV-2 neuroinvasion and persistence in the olfactory system. bioRxiv [Preprint] 10.1101/2020.11.18.388819. (accessed on February 27, 2021). [DOI] [Google Scholar]
- Demski L.S. (1993). Terminal nerve complex. Acta Anat (Basel). 148(2–3), 81–95. [DOI] [PubMed] [Google Scholar]
- Dubé M., Le Coupanec A., Wong A.H.M., Rini J.M., Desforges M., and Talbot P.J. (2018). Axonal Transport Enables Neuron-to-Neuron Propagation of Human Coronavirus OC43. J Virol. 92(17), 404–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellul M.A., Benjamin L., Singh B., Lant S., Michael B.D., Easton A., et al. (2020). Neurological associations of COVID-19. Lancet Neurol. 19(9), 767–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennes L. (1987). The nervus terminalis in the mouse: light and electron microscopic immunocytochemical studies. Ann N Y Acad Sci. 519,165–173. [DOI] [PubMed] [Google Scholar]
- Jiang R. D., Liu M. Q., Chen Y., Shan C., Zhou Y. W., Shen X. R., et al. (2020). Pathogenesis of SARS-CoV-2 in Transgenic Mice Expressing Human Angiotensin-Converting Enzyme 2. Cell. 182(1), 50–58.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z.W., Cho K.H., Shibata S., Yamamoto M., Murakami G., and Rodríguez-Vázquez J.F. (2019). Nervus terminalis and nerves to the vomeronasal organ: a study using human fetal specimens. Anat Cell Biol. 52(3), 278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.H., Patel L., Tobet S.A., King J.C., Rubin B.S., and Stopa E.G. (1999). Gonadotropin-releasing hormone immunoreactivity in the adult and fetal human olfactory system. Brain Res. 826(2), 220–229. [DOI] [PubMed] [Google Scholar]
- Klingenstein M., Klingenstein S., Neckel P.H., Mack A.F., Wagner A.P, Kleger A., et al. (2021). Evidence of SARS-CoV2 Entry Protein ACE2 in the Human Nose and Olfactory Bulb. Cells Tissues Organs E-pub. doi: 10.1159/000513040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsell O. (1918). Nervus terminalis: mammals. J Comp Neurol. 30, 3–68. [Google Scholar]
- Larsell O. (1950). The nervus terminalis. Ann Otol Rhinol Laryngol. 59(2), 414–438. [DOI] [PubMed] [Google Scholar]
- Leist S.R., Dinnon K.H., Schäfer A., Tse L.V., Okuda K., Hou Y.J., et al. (2020). A Mouse-Adapted SARS-CoV-2 Induces Acute Lung Injury and Mortality in Standard Laboratory Mice. Cell. 183 (4),1070–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Liu T., Yang N., Han D., Mi X., Li Y., et al. (2020). Neurological manifestations of patients with COVID-19: potential routes of SARS-CoV-2 neuroinvasion from the periphery to the brain. Front Med. 14(5), 533–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang F. (2020). Sustentacular Cell Enwrapment of Olfactory Receptor Neuronal Dendrites: An Update. Genes (Basel). 11(5), 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matschke J., Lütgehetmann M., Hagel C., Sperhake J.P., Schröder A.S., Edler C., et al. (2020). Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 19(11), 919–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt J., Radke J., Dittmayer C., Franz J., Thomas C., Mothes R., et al. (2020). Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci. doi: 10.1038/s41593-020-00758-5 [DOI] [PubMed] [Google Scholar]
- Mukerji S.S., and Solomon I.H. (2020). What can we learn from brain autopsy in COVID-19? Neurosci Lett. 135528. doi: 10.1016/j.neulet.2020.135528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nampoothiri S., Sauve S., Ternier G., Fernandois D., Coelho C., Imbernon M., et al. (2020). The hypothalamus as a hub for putative SARS-CoV-2 brain infection. bioRxiv [Preprint] doi: 10.1101/2020.06.08.139329. (accessed on February 27, 2021) [DOI] [Google Scholar]
- Natoli S., Oliveira V., Calabresi P., Maia L.F., and Pisani A. (2020). Does SARS-Cov-2 invade the brain? Translational lessons from animal models. Eur J Neurol. 9,1764–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netland J., Meyerholz D.K., Moore S., Cassell M., and Perlman S. (2008). Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 82(15), 7264–7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelschläger H.A., Buhl E.H., and Dann J.F. (1987). Development of the nervus terminalis in mammals including toothed whales and humans. Ann N Y Acad Sci. 519, 447–464. [DOI] [PubMed] [Google Scholar]
- Oladunni F. S., Park J. G., Pino P. A., Gonzalez O., Akhter A., Allué-Guardia A., et al. (2020). Lethality of SARS-CoV-2 infection in K18 human angiotensin-converting enzyme 2 transgenic mice. Nat Commun. 11(1), 6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson A.A. (1941). The development of the nervus terminalis in man. J Comp Neurol. 75, 39–66. [Google Scholar]
- Rathnasinghe R., Strohmeier S., Amanat F., Gillespie V.L., Krammer F., García-Sastre A., et al. (2020). Comparison of transgenic and adenovirus hACE2 mouse models for SARS-CoV-2 infection. Emerg Microbes Infect. 1, 2433–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway S.H., Demski L.S., Bullock T.H., and Schwanzel-Fukuda M. (1987). The terminal nerve in odontocete cetaceans. Ann N Y Acad Sci. 519, 201–212. [DOI] [PubMed] [Google Scholar]
- Schwanzel-Fukuda M., Morrell J. I., and Pfaff D. W. (1986). Localization of choline acetyltransferase and vasoactive intestinal polypeptide-like immunoreactivity in the nervus terminalis of the fetal and neonatal rat. Peptides. 7(5), 899–906. [DOI] [PubMed] [Google Scholar]
- Schwanzel-Fukuda M., Garcia M.S., Morrell J.I., and Pfaff D.W. (1987). Distribution of luteinizing hormone-releasing hormone in the nervus terminalis and brain of the mouse detected by immunocytochemistry. J Comp Neurol. 255(2), 231–244. [DOI] [PubMed] [Google Scholar]
- Solomon T. (2021). Neurological infection with SARS-CoV-2 - the story so far. Nat Rev Neurol. 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikellis C., and Thomas M.C. (2012). Angiotensin-Converting Enzyme 2 (ACE2) Is aKey Modulator of the Renin Angiotensin System in Health and Disease. Int J Pept. 2012:256294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Riel D., Verdijk R., and Kuiken T. (2015). The olfactory nerve: a shortcut for influenza and other viral diseases into the central nervous system. J Pathol. 235(2), 277–287. [DOI] [PubMed] [Google Scholar]
- von Bartheld C.S. (2004). The terminal nerve and its relation with extrabulbar “olfactory” projections: lessons from lampreys and lungfishes. Microsc Res Tech. 65(1–2),13–24. [DOI] [PubMed] [Google Scholar]
- Winkler E. S., Bailey A. L., Kafai N. M., Nair S., McCune B. T., Yu J., et al. (2020). SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nat Immunol. 21(11), 1327–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirsig C.R., and Leonard C.M. (1986). Acetylcholinesterase and luteinizing hormone-releasing hormone distinguish separate populations of terminal nerve neurons. Neuroscience, 19(3), 719–740. [DOI] [PubMed] [Google Scholar]
- Ye Q., Zhou J., Yang G., Li R.-T., He Q., Zhang Y., et al. (2020). SARS-CoV-2 infection causes transient olfactory dysfunction in mice. bioRxiv [Preprint] doi: 10.1101/2020.11.10.376673. (accessed on February 26, 2021). [DOI] [Google Scholar]
- Zhang A.J., Lee A.C., Chu H., Chan J.F., Fan Z., Li C., et al. (2020). SARS-CoV-2 infects and damages the mature and immature olfactory sensory neurons of hamsters. Clin Infect Dis. 15:ciaa995. doi: 10.1093/cid/ciaa995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J., Wong L.R., Li K., Verma A.K., Ortiz M., Wohlford-Lenane C., et al. (2021). COVID-19 treatments and pathogenesis including anosmia in K18-hACE2 mice. Nature 589, 603–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B., Thao T.T.N., Hoffmann D., Taddeo A., Ebert N., Labroussaa F., et al. (2020). SARS-CoV-2 spike D614G variant confers enhanced replication and transmissibility. bioRxiv [Preprint]. doi: 10.1101/2020.10.27.357558. (accessed on February 26, 2021). [DOI] [Google Scholar]
- Zubair A.S., McAlpine L.S., Gardin T., Farhadian S., Kuruvilla D.E., and Spudich S. (2020). Neuropathogenesis and Neurologic Manifestations of the Coronaviruses in the Age of Coronavirus Disease 2019: A Review. JAMA Neurol. 77(8), 1018–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.