Figure 1.
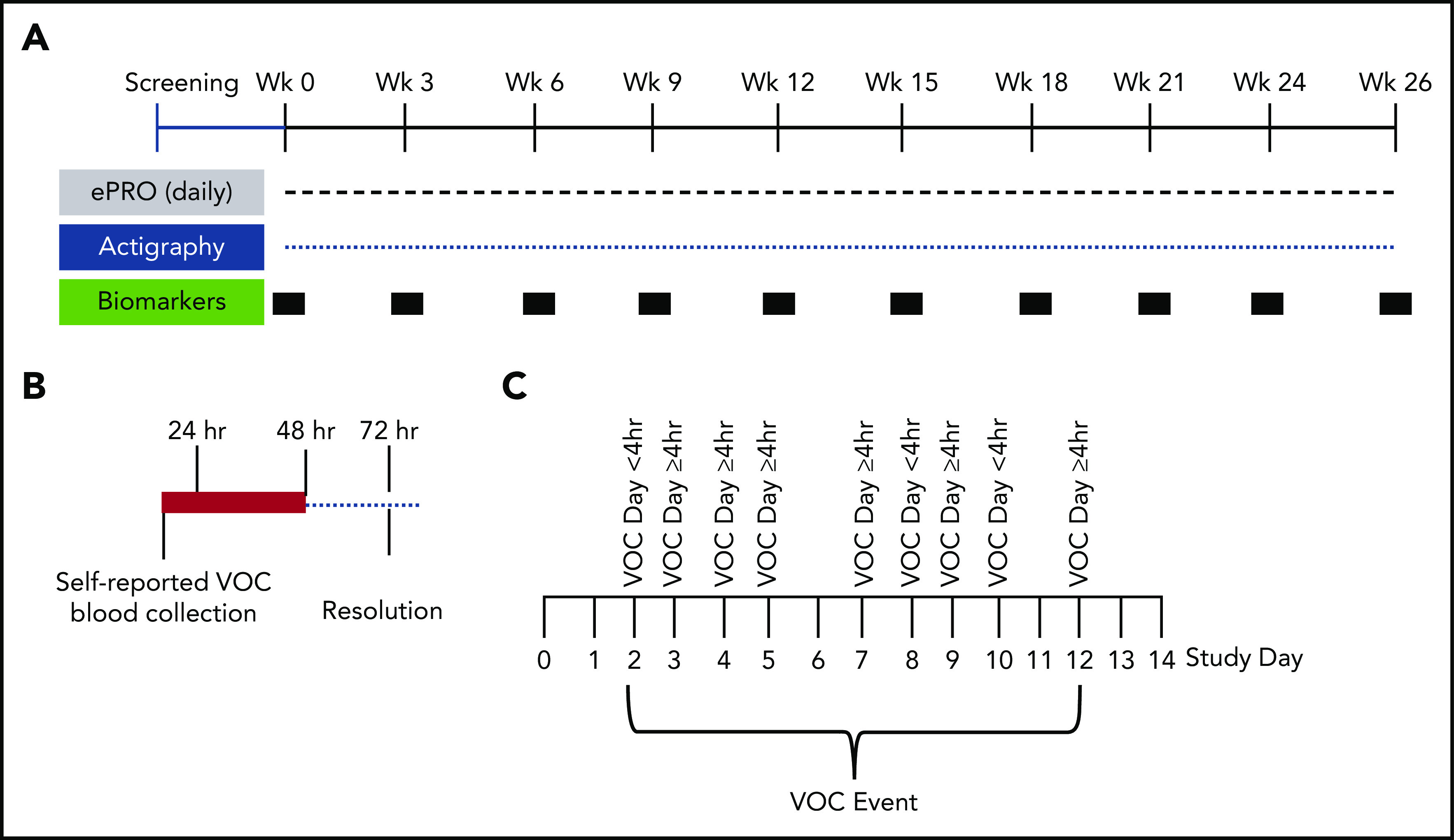
Schematic of study design. (A) Study design for the 6-month longitudinal analysis of SCD patients (with and without hydroxyurea therapy) to document the natural history of self-reported VOCs. This innovative at-home study design used the ePRO tool for self-reported daily pain, fatigue, function, and medication. Pain, functionality, fatigue, and medications were recorded by using the e-diary. Participants recorded their experience of pain crisis in a 24-hour recall period. The ePRO documented real-time self-reported VOC days that tracked the patient’s treatment decisions (at home vs emergency department or hospital). Blood samples for biomarkers and clinical laboratory tests were drawn by a mobile phlebotomist in the subject’s home every 3 weeks during non-VOC periods to establish longitudinal baseline values. Activity was monitored continuously throughout the study by using the Philips Actiwatch Spectrum. (B) Blood collections by mobile phlebotomists were triggered within 24 hours and 48 hours of self-reported VOCs, with follow-up collections 72 hours after the VOC resolution. These collections occurred at the subject’s home or at the medical facility where the subject sought treatment. Baseline, non-VOC samples were restarted 2 weeks after resolution of the VOC (if blood volume limits were not exceeded). (C) Depicted is an example of a VOC event. A VOC event was described as a sequence of VOC days that can also include intermittent days with no pain crisis. The VOC event resolves when there are no VOC days for 2 consecutive study days.
