Figure 2.
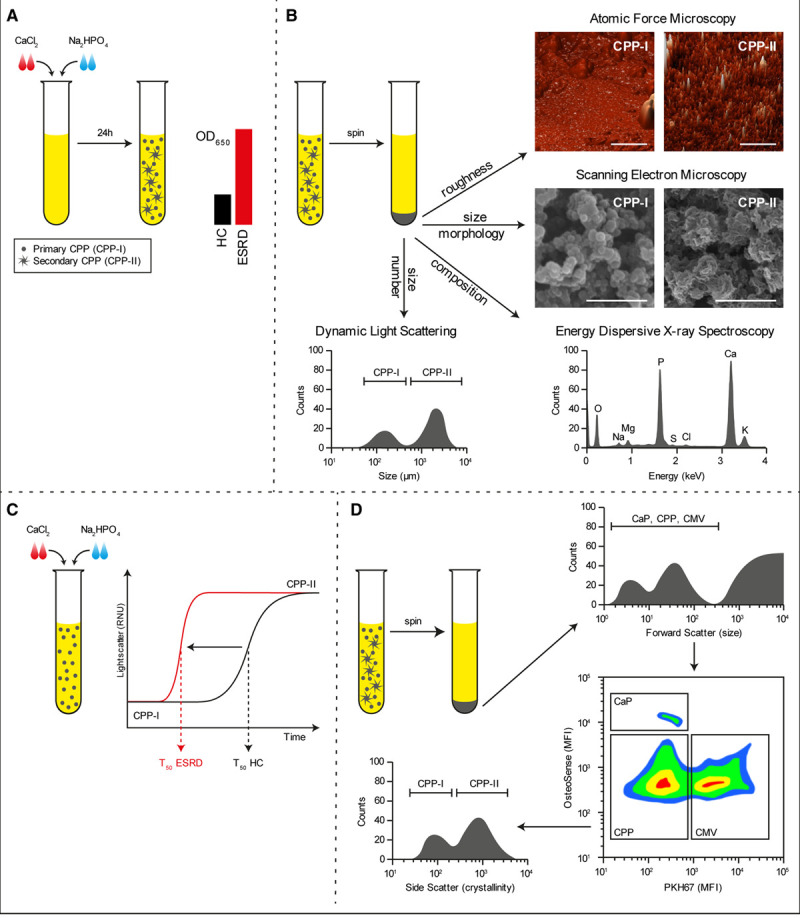
Methods to detect calciprotein particles (CPPs) in clinical samples. Supersaturation of serum with calcium chloride (CaCl2) and sodium diphosphate (Na2HPO4) followed by incubation under culture conditions for 24 h causes the formation of CPPs that can be measured by absorbance at 650 nm. In disease conditions wherein CPP levels are increased, the OD650 readings increase (A). Alternatively, CPPs can be pelleted by centrifugation and investigated by dynamic light scattering to assess particle size, electron and atomic force microscopy to assess morphology, or elemental analysis (EDX) to assess mineral constituent (B). Supersaturation of serum is also used to measure the one-half maximal transition time needed for amorphous-to-crystalline transition (T50). An increased serum propensity for secondary CPP formation is observed as a reduction in T50 (C). A novel flow cytometry-based technique allows for the direct quantification of CPP levels in serum. Here, serum precipitates are labeled with a combination of a fluorescent bisphosphonate (osteoSense) and a fluorescent membrane-intercalating dye (PKH67) and separated based on size, calcium phosphate content, and the presence of membranous lipids. CPPs are observed as OsteoSense+/PKH67− events that fluoresce dim compared to calcium phosphate crystal (CaP) crystals. CPPs are further characterized as primary- or secondary CPPs based on crystallinity (D). CMVs indicates calcifying microvesicles; ESRD, end-stage renal disease sample; HC, healthy control sample; MFI, mean fluorescence intensity; and OD, optical density.
