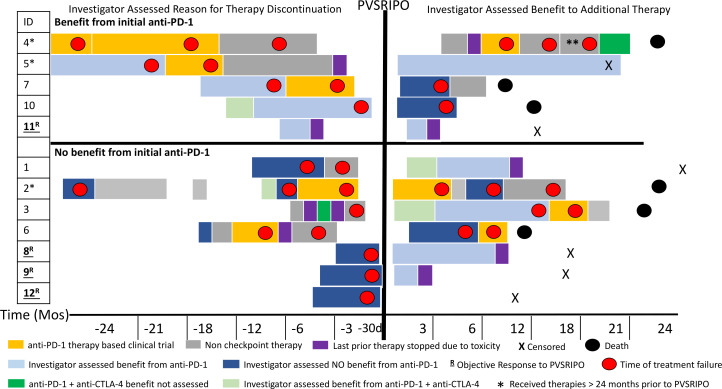
Figure 2.
Event history plot. Visual depiction of patients’ therapies prior to and after PVSRIPO. Patients are grouped on the basis of physician-assessed benefit of initial anti-PD-1 therapy, noted on the left side of the panel. Left top panel shows patients who had an initial benefit from anti-PD-1 (programmed cell death protein 1) therapy while left bottom panel shows patients who did not have initial benefit from anti-PD-1 therapy. The X-axis shows time in months before PVSRIPO and then months after PVSRIPO except where d is indicated. The duration of treatment is indicated by length of the block,;red dots indicate time of disease progression. Light blue is assessed benefit to PD-1; dark blue is no benefit to PD-1; dark green is PD-1 plus anti-CTLA-4 (cytotoxic T -lymphocyte-associated protein 4) therapy for which response could not be assessed (limited time or limited exposure); light green is benefit from PD-1 plus anti-CTLA-4 therapy; and purple indicates therapy stopped due to toxicity. Patients were censored (X) and deceased (black circle). Non-checkpoint therapies included BRAF (v-raf murine sarcoma viral oncogene homolog B1)/MEK (mitogen-activated protein kinase kinase) inhibition, radiation, T-VEC, and surgery. Orange indicates other clinical trials in which patients were enrolled that included anti-PD-1 as part of the experimental regimen. The single asterisk (patients 4, 5, and 2) indicates patients who received therapies for the treatment of metastatic melanoma greater than 24 months prior to PVSRIPO. The double asterisk indicates patient 4 was on anti-PD-1-based therapy plus chemotherapy. Patient number in the trial is listed under ID; patients underlined and bolded with R are those who had an objective response to PVSRIPO. d, day; PD-1, programmed cell death protein 1; T-VEC, talimogene laherparepvec.