Description
A 49-year-old man with Down syndrome and obesity was admitted to our institution with a diagnosis of COVID-19 pneumonia, confirmed by tomography and real-time PCR test for SARS-CoV-2. In less than 48 hours, the patient was intubated at the intensive care unit (ICU) due to severe acute respiratory distress syndrome. Later, he developed ventilator-associated pneumonia followed by septic shock and was started with meropenem pending culture results. Although the patient had persistent fever, there was an overall improvement in his respiratory status, so he was transferred to the medical floor. Nevertheless, under the suspicion of a bloodstream infection, meropenem was restarted and caspofungin was added as empiric treatment. Two days later, the results from blood and bronchial aspirate cultures were obtained that showed multidrug-resistant Pseudomonas aeruginosa. Given the antibiogram result, meropenem was stopped and gentamicin and colistin started. However, despite the aforementioned treatment, he continued with fever episodes; thus, greater coverage was included with ceftazidime/avibactam. The fever never stopped, and due to suspected complicated pseudomonal infection, meropenem was added for the third time doubling the dose, and caspofungin was stopped. After 4 days of meropenem use, there was small pinpoint vesicular skin lesions in the upper trunk. A mild adverse reaction was suspected. However, a few days later, he suddenly developed respiratory distress and altered mental status. Physical examination showed diffuse purpuric rash with the involvement of 60% body surface area, anterior cervical chain lymphadenopathy, and facial and lower limb oedemas (figure 1). Laboratory workup showed a significant eosinophilic predominant leucocytosis and elevated creatinine of 10.5 mg/dL showing acute kidney failure (figure 2). No liver impairment was seen. Chest X-ray showed bilateral pleural effusion. Given these findings, drug rash with eosinophilia and systemic symptoms (DRESS) syndrome was suspected. The case was approached using the Registry of Severe Cutaneous Adverse Reactions score, a registry of severe cutaneous adverse reactions that validates the diagnosis of potential DRESS cases. The score was a total of 6 points, making it a definite case.1 2 After the assessment, all antibiotics were stopped and the patient was treated with methylprednisolone 80 mg intravenously every six hours, tapering the dose to half after 5 days.3 4 Despite the patient’s severe organ involvement, a conservative approach was taken, with continuing to the monitoring of his renal function and laboratory markers every day.5 6 After 4 days, the patient showed laboratory and dermatological improvement, and a few days later, he was discharged home. At outpatient follow-up 2 weeks later, cutaneous and systemic symptoms cleared, and all laboratory tests were normal. Twelve weeks later, the analysis showed positivity for IgG for Epstein-Barr virus (Epstein-Barr virus nuclear antigen and viral capsid antigen) and IgG for cytomegalovirus.
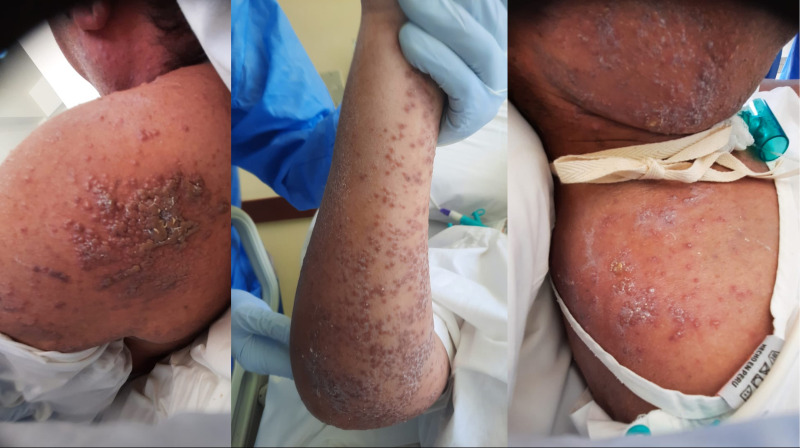
Figure 1.
Cutaneous presentation in our patient with suspected drug rash with eosinophilia and systemic symptoms syndrome. Diffuse purpuric confluent morbilliform skin lesions with follicular accentuation and raised borders on shoulder and arms. Scaling areas were covering the face.
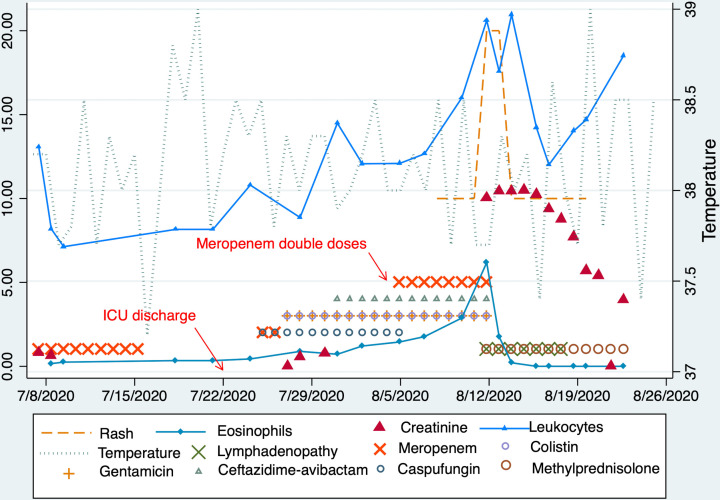
Figure 2.
Drug rash with eosinophilia and systemic symptoms (DRESS) syndrome course in our patient. We describe the timeline of antibiotics, specially meropenem (pointing to when the dose was doubled) and its relationship with the appearance of clinical and laboratory manifestation of DRESS. Eosinophils and leucocytes in ×103 cell/µL and creatinine in mg/dL. IgG for Epstein-Barr virus nuclear antigen was 47.9 U/mL (positive >3) and for viral capsid antigen was 127.9 U/mL (positive >15), and IgG for cytomegalovirus was in the ratio of 14.8 (reactive >1.1). ICU, intensive care unit.
Patient’s perspective.
When he was discharged from the intensive care unit (ICU), I did not imagine his condition was about to be critical. Even though I notice he was discharged with fever episodes, I thought that with rehabilitation therapy, he was going to get better, and we could go home. He was at the ICU for a long time, so I knew there was the possibility of nosocomial infection. When the results of this deadly bacteria came from the cultures, I felt kind of relieved because they started all these antibiotics. But many other events came such as his nasojejunal tube, the constant secretions on his tracheostomy tube and the possibility of a tracheoesophageal fistula. When the meropenem was restarted and increased in dose, I hoped to notice a small change for the best. Then, drug rash with eosinophilia and systemic symptoms syndrome happened, and it was an extreme event that scared me a lot. I got desperate because everyone told me this was a very severe disease, and that life expectancy was low. I understood that, and I wished to take him home so that he could die with his family. I saw him suffer so much, not only because of the rash that was in his body, but also because he was so swollen, he could not even move, and it was even hard for him to urinate. When the doctors stopped the antibiotics, all I could do was wait. Fortunately, after a few days, I finally saw a glimpse of hope. He was getting better, and I took him home. But, even at home, I was scared to give him the antibiotics the doctors gave me when he was discharged, and I think I fell this way with all his medications. I’m a little scared that something like this could happen again. I hope he can be the person he used to be and that can go to work eventually because he loves his job. I know that by having Down syndrome, he would probably have a few years left, but I want to continue to enjoy my life with him as much as I can.
Learning points.
The overuse of antibiotics in critically ill patients during a course of severe COVID-19 pneumonia can lead to idiosyncratic reactions that put the patient’s life in danger. Take an individualised approach when dealing with secondary bacterial infections such as complications of COVID-19 pneumonia.
Drug rash with eosinophilia and systemic symptoms (DRESS) syndrome is a rare, life-threatening condition with a mortality rate of around 10% that requires a vigilant diagnosis. The hallmarks are fever, lymphadenopathy, eosinophilia, skin eruption and organ involvement. The damage to organs can be very severe, so to reach a correct and rapid diagnosis, we recommend using the Registry of Severe Cutaneous Adverse Reactions score system. The score is based on clinical manifestations and laboratory findings. According to the score, it can be excluded, possible, probable and definite.
Management of DRESS should include withdrawal of all inciting medications, supportive measures and early start of corticosteroids when there is evidence of organ damage.
Acknowledgments
The authors thank Dr Cynthia Diaz for the pictures.
Footnotes
Contributors: LM, HTZ and AGG worked on the acquisition of the data for the case, drafting the case and made the final approval of the last version.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (dress): an original multisystem adverse drug reaction. results from the prospective RegiSCAR study. Br J Dermatol 2013;169:1071–80. 10.1111/bjd.12501 [DOI] [PubMed] [Google Scholar]
- 2.Qadri I, Zeng X, Guo R, et al. Acute interstitial nephritis and dress syndrome without eosinophilia associated with cefepime. BMJ Case Rep 2017;2017. 10.1136/bcr-2017-221401. [Epub ahead of print: 03 Aug 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cacoub P, Musette P, Descamps V, et al. The dress syndrome: a literature review. Am J Med 2011;124:588–97. 10.1016/j.amjmed.2011.01.017 [DOI] [PubMed] [Google Scholar]
- 4.Chen Y-C, Chiu H-C, Chu C-Y. Drug reaction with eosinophilia and systemic symptoms: a retrospective study of 60 cases. Arch Dermatol 2010;146:1373–9. 10.1001/archdermatol.2010.198 [DOI] [PubMed] [Google Scholar]
- 5.Husain Z, Reddy BY, Schwartz RA. Dress syndrome: Part II. management and therapeutics. J Am Acad Dermatol 2013;68:718–20. 10.1016/j.jaad.2013.01.032 [DOI] [PubMed] [Google Scholar]
- 6.Cardoso CS, Vieira AM, Oliveira AP. Dress syndrome: a case report and literature review. Case Reports 2011;2011:bcr0220113898. 10.1136/bcr.02.2011.3898 [DOI] [PMC free article] [PubMed] [Google Scholar]