Figure 1.
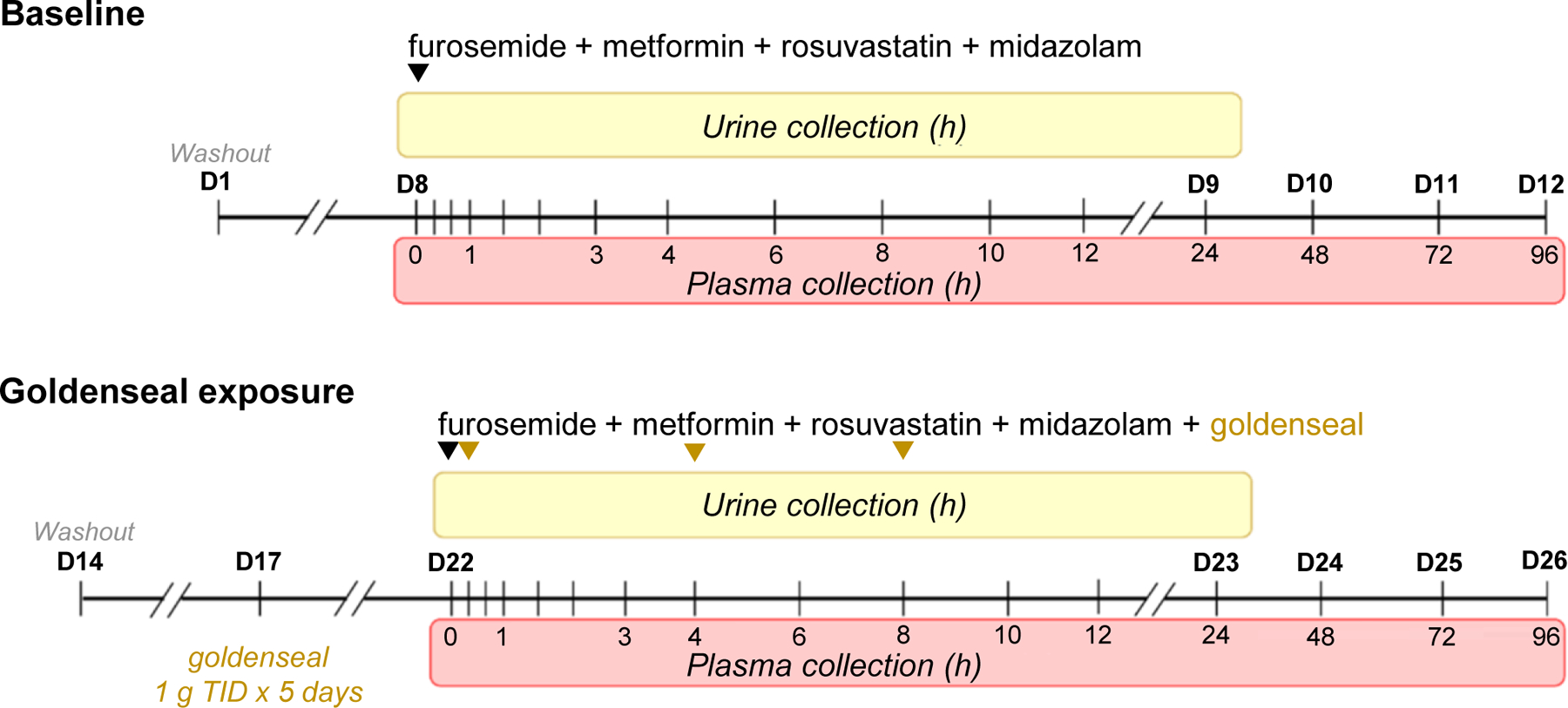
Healthy adult volunteers were enrolled in a two-arm, open-label, fixed sequence, crossover study. Arm 1 (baseline) entailed administration of a transporter probe cocktail containing 1 mg furosemide, 50 mg metformin, 10 mg rosuvastatin, and 2.5 mg midazolam (black inverted triangle). A minimum of 9 days of washout ensured no residual analytes. Arm 2 (goldenseal exposure) entailed administration of 1 g goldenseal thrice daily for 5 consecutive days to ensure berberine reached steady state (t1/2 28 h).49 On day 6 of Arm 2, participants were administered the oral probe cocktail with 1 g goldenseal; two additional doses of goldenseal (1 g) were administered in 4-hour increments (gold inverted triangles). During both arms, plasma was collected at designated times (shaded in red) up to 96 hours post-cocktail administration; urine was collected in 12-hour intervals (0–12 and 12–24; shaded in yellow) immediately following cocktail administration.
