Abstract
Background
The introduction of a robot into the surgical suite changes the dynamics of the work-system, creating new opportunities for both success and failure. An extensive amount of research has identified a range of barriers to safety and efficiency in Robotic Assisted Surgery (RAS), such as communication breakdowns, coordination failures, equipment issues, and technological malfunctions. However, there exists very few solutions to these barriers. The purpose of this review was to identify the gap between identified RAS work-system barriers and interventions developed to address those barriers.
Methods
A search from three databases (PubMed, Web of Science, and Ovid Medline) was conducted for literature discussing system-level interventions for RAS that were published between January 1, 1985 to March 17, 2020. Articles describing interventions for systems-level issues that did not involve technical skills in RAS were eligible for inclusion.
Results
A total of 30 articles were included in the review. Only seven articles (23.33%) implemented and evaluated interventions, while the remaining 23 articles (76.67%) provided suggested interventions for issues in RAS. Major barriers identified included disruptions, ergonomic issues, safety and efficiency, communication, and non-technical skills. Common solutions involved team training, checklist development, and workspace redesign.
Conclusion
The review identified a significant gap between issues and solutions in RAS. While it is important to continue identifying how the complexities of RAS affect operating room (OR) and team dynamics, future work will need to address existing issues with interventions that have been tested and evaluated. In particular, improving RAS-associated non-technical skills, task management, and technology management may lead to improved OR dynamics associated with greater efficiency, reduced costs, and better systems-level outcomes.
Keywords: Human factors, Checklists, Communication, Simulation, Team training
Robotic-Assisted Surgery (RAS) has revolutionized many procedures, with popularity among surgeons and patients leading to a tripling of robotic-assisted cases over the past decade [1]. In fact, one million RAS cases were performed between 2009 and 2012 [2]. The benefits of RAS compared to open surgery include less postoperative pain, reduced blood loss, shorter hospital stays, and quicker recovery times [3]. The improved dexterity and precision provided by the robot instruments allows for a minimally invasive approach to some procedures, particularly in the pelvis, that are less suitable for a more traditional laparoscopic approach [4]. However, safety incidents in RAS may be double that of traditional open surgery [5], and only recently have systems engineering studies begun to explore the causes and strategies for mitigation [6–13].
In addition to changing basic surgical tasks and skills (which are generally the focus of attention when exploring opportunities to improve RAS), RAS implementation introduces a range of non-technical challenges which can have important performance implications. The isolation of the surgeon at the console and separation from the rest of the team necessitates changes in coordination, communication, and teamwork [14, 15]. This solution also increases the demand on verbal communication [7, 12] which is already one of the most frequently cited causes of procedural error [16] and surgical injury [17, 18]. Difficulties with port placement, docking, instrument changes and unnecessary procedural steps contribute significantly to operative time [19] exacerbating specific complications of anesthesia that arise from placing the patient in the steep Trendelenburg (head-down) position [20, 21]. The size of the robot and associated technologies exacerbate layout issues that are known to contribute to clutter, obstructions, congestion from equipment and displays, disorganization of tubes and lines, unnecessary movement, distractions, team performance [22], infection risk, increased risk of accidental disconnection of devices [23], and slips, trips and falls [24]. RAS has particularly acute effects on equipment congestion, the movement paths of staff, and the safe positioning of data and power cables [9]. These issues can persist due to the lack of organizational resources to redesign ill-equipped operating rooms (OR) [25]. Thus, RAS increases task demands for the whole OR team and increases reliance on teamwork and communication while exacerbating already challenging workspace issues. These can adversely impact surgical outcomes [26].
We sought to systematically review evidenced-based approaches that had been used to understand and address these issues in RAS. Outside of RAS, frequent attempts have been made to redesign multiple systems-level components, such as tasks, workspaces, and team training to improve safety and performance. Checklists have been particularly influential on teamwork and communication in the OR [27] and have been associated with improving outcomes in surgery [28]. Non-technical skills, which are those that require interpersonal and cognitive abilities to complement technical skills [29] (e.g., situational awareness, decision making, communication, teamwork, and leadership) [26] have been demonstrated to play a significant role in surgical outcomes alongside surgical technique. Although some literature exists on interventions developed to improve work-system function in RAS, the majority of the literature focuses on the identification of barriers. Given the imbalance between the discovery of issues and the creation and implementation of interventions to alleviate those issues, we aimed to conduct a systematic review to better understand existing interventions developed in hopes of reducing challenges in RAS.
Materials and methods
Using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses [30] (PRISMA) methodology, a systematic review was conducted to examine all studies reporting systems-level interventions in RAS from the introduction of the first surgical robot in 1985 [31] through January 2020. Institutional review board (IRB) approval and written consent were not required for this study.
Inclusion criteria
Peer-reviewed articles in the English language were included in the review if the study focused on identifying, implementing, or implementing and evaluating interventions aimed to reduce systems-level issues in RAS.
Exclusion criteria
Articles were excluded if the publication was a literature or narrative review, an abstract, a poster or conference presentation, a commentary, an editorial, a viewpoint, the focus of the study was on technical skills or the design of the robotic console, or the outcome measures of the study were focused on clinical methods.
Search criteria
The search was conducted on March 17, 2020 using PubMed, Web of Science, and Ovid Medline. The search strategy involved locating articles that focused on RAS, systemic issues posed during RAS, and suggested or implemented and evaluated interventions to improve systemic issues. The following keywords were used in conjunction with “robotic surgery” OR “robotic-assisted surgery”: “interventions,” “solutions,” “teamwork”, “communication,” “coordination,” “Human Factors,” “Ergonomics,” “checklist,” “checklists,” “non-technical skills,” “training,” “improve,” “development,” “assessment,” “task design,” “task analysis,” “workspace,” “lean,” “six sigma,” “quality improvement,” “implementation,” “workflow,” and “workload”.
Selection methods
Searches from each of the three databases were uploaded into Rayyan QCRI, a web and mobile application for systematic reviews [32]. Duplicate records were discarded prior to the review and categorization of each article. Two reviewers (FK, EC) independently reviewed and categorized each of the non-duplicate studies with arbitration by a third reviewer (TC) when necessary. The review and categorization process began with the title and abstracts and were either included or excluded based on the inclusion and exclusion criteria. The full text of the remaining articles was reviewed if the abstract provided information about system-level issues in RAS. Following the review of the included articles, two reviewers (FK, TC) organized the articles into eight categories based on the non-technical areas that were addressed. The articles were then further categorized by two reviewers (FK, EC) based on the type of intervention discussed or tested (e.g., checklists) and then by year, title, authors, abstract, issue addressed, and intervention (suggested, implemented, or implemented and evaluated, see Table 1).
Table 1.
Studies included in the systematic review
| Reference | Problem addressed | Intervention Implementation (S: suggested, E: evaluated) | Intervention category | Intervention details | Intervention challenges |
|---|---|---|---|---|---|
| Ahmad [25] | Impact of workspace congestion on workflow during robotic-assisted surgery | S | Environment | Redesigning the operating room layout | Proposed interventions may only be applicable to some specialties/ may only be ideal for only certain OR types |
| Ahmed [37] | “Assess potential hazards in robotic-assisted urological surgery” | S | Checklist | A 22-item checklist was developed after analysis of systemic-level issues using the Healthcare Failure Mode and Effects Analysis | n/a |
| Allers [38] | “Interruptions during robotic-assisted surgery” | S | Environment | Redesigning the operating room layout and providing training to surgical team members | n/a |
| Catchpole [14] | Safety and efficiency during robotic-assisted surgery | S | Workflow | Improving system-level issues such as communication, coordination, and training | n/a |
| Collins [29] | The need for a proficiency-based standardized curriculum that includes both technical and non-technical training | S | Training | Simulation training for non-technical skills and team training | n/a |
| Craven [39] | Upper limb ergonomic strain of the surgeon on console | S | Ergonomics | Ergonomic modifications to the console workstation and posture | Ergonomic modifications may not withstand the duration of the procedure and may not be applicable for all surgeons |
| Dru [40] | Identification of flow disruptions that can lead to patient harm, surgical inefficiency, frustrations, communication breakdown, and longer operating times | S | Training | Technical and non-technical simulation training for surgical team members | n/a |
| Franasiak [41] | Ergonomic positioning of the surgeon at the console | E | Training | Training on ergonomic modifications to the console workstation and posture | Reports of strain are subjective |
| Jain [8] | Flow disruptions during robotic-assisted surgery | S | Training, Briefings | Teamwork training, preoperative briefings, and improvement of equipment maintenance and use | n/a |
| Jing [33] | Effectiveness of a checklist for robotic-assisted radical prostatectomies | E | Checklist | Implementation of a checklist for robotic-assisted prostatectomies | n/a |
| McCarroll [34] | Evaluate the effectiveness of a checklist for robotic-assisted gynecological procedures | E | Checklist | Implementation of a computerized checklist for robotic-assisted surgery | OR staff members were not as motivated to use the checklist if a specific nurse was not present, potentially resulting in a pseudo-Hawthorne effect |
| Myklebust [42] | Assess the technical/non-technical elements of teamwork that enhance efficiency and flow, and patient safety | S | Training, Checklist | Simulation-based team training, checklists, and team leader designation during robotic-assisted surgery | n/a |
| Raheem [43] | Communication during robotic-assisted surgery | S | Communication, Training | Standardized communication system to improve exchange of information | n/a |
| Randell [44] | “Integration of robotic-assisted surgery into routine practice” | S | Environment, Teamwork, Training | Operating room staff engagement, training, and operating room setup | n/a |
| Schiff [45] | “Quality of communication and surgical outcomes in robotic-assisted surgery” | S | Communication, Teamwork | A systematic approach to introducing inexperienced operating room staff into robotic-assisted surgery | n/a |
| Schuessler [46] | Assess the non-technical aspects of robotic-assisted surgery that contributes to improved patient and system outcomes | S | Training | Team-focused training to improve the practice of robotic-assisted surgery | n/a |
| Sexton [47] | Investigate the impact of anticipation as a measure of efficiency in robotic-assisted surgery | S | Teamwork | Develop an understanding of team dynamics and familiarity to improve team efficiency during robotic-assisted surgery | n/a |
| Song [48] | Complications and obstacles that occur during RAS | Ea | Checklist | Development of a checklist to improve patient safety during robotic-assisted surgery | n/a |
| Souders [49] | Identify flow disruptions that contribute to decreased efficiency, errors, and suboptimal patient outcomes | S | Environment | Modification to the operating room setup for robotic-assisted abdominal sacrocolpopexy surgeries to improve efficiency | n/a |
| Tiferes [50] | Team communication during robotic-assisted surgery | S | Communication | Improve team communication through nonverbal actions and usability testing of surgical equipment | n/a |
| Tiferes [10] | The gap in literature addressing the analysis of team activities during robotic-assisted surgery | S | Teamwork, Environment | Modification to the operating room setup for robotic-assisted surgery to improve safety and team efficiency | n/a |
| Tsafrir [51] | Quality of verbal and auditory communication in a robotic operating room | E | Technology | Evaluation of wireless audio headsets in a robotic operating room to improve the quality of communication | Ambient noise levels are not eliminated because the headset covers one ear |
| Van’t Hullenaar [52] | Ergonomic positioning of the first assistant during robotic-assisted surgery | S | Ergonomics | Implementation of ergonomic modifications for the first assistant during robotic-assisted surgery | n/a |
| Van’t Hullenaar [53] | Address possible deficiencies in training when optimizing the ergonomics and positioning of the surgeon | E | Training | Development of an ergonomic training program to provide instructions for ergonomic setup of the robotic console | Optimal interpretation and accuracy of the ergonomic setup are not guaranteed |
| Wastler [54] | “Communication and patient safety during robotic-assisted surgery” | S | Checklist | Development of a checklist to improve “patient safety during robotic-assisted surgery” | n/a |
| Weber [55] | Effect of flow disruptions on workload and performance | S | Training | Implementation of team training and strategies to improve nonverbal communication, situational awareness, and team coordination | n/a |
| Weigl [56] | Severity and impact of flow disruptions on the perception of teamwork | S | Communication. Teamwork, Environment | Improving system-level issues such as communication, coordination, operating room setup and management and training | n/a |
| Yu [11] | Assess ergonomics and workload for the first assistant and surgeon on console | S | Technology | Introduce wearable intraoperative motion tracking sensors to identify ergonomic risks in the operating room during robotic-assisted surgery | A trained researcher is required to analyze motion tracking data and sensors are not able to “differentiate whether musculoskeletal efforts of static posture are demanding or not” |
| Zattoni [57] | Non-technical errors during the conversion of robot-assisted to open radical prostatectomy | E | Guidelines | Development of guidelines for conversion from robotic-assisted radical prostatectomy to open radical prostatectomy | The guidelines were developed for a specific type of surgery and may not account for other surgical complications and patient factors |
| Zattoni [58] | Evaluate the impact of training and checklists on improving teamwork during open conversion from robotic-assisted partial nephrectomy | E | Training | Evaluation of a standardized training and institutional checklist on improving teamwork during complications requiring open conversion from robotic-assisted partial nephrectomy | The simulated environment produces shorter conversion times and may not accurately represent real-life experiences during robotic-assisted surgery |
Evaluation of intervention was discussed, but no data were presented
Results
Search results from PubMed, Web of Science, and Ovid Medline returned 4630 articles that contained any one of the search terms AND either the key term “robotic surgery” or “robotic-assisted surgery”. Of the 4630 articles, 1868 were removed due to duplication. The abstracts of the remaining 2762 articles were reviewed for selection. At the conclusion of this process, 2727 articles were excluded and the full text of the remaining 42 articles were reviewed for selection. Of the 42 articles, 12 were excluded due to the following reasons: the focus of the study was on technical skills (n = 4), the focus of the study was on the design of the robotic system (n = 1), a systems-level intervention was not provided (n = 4), the information provided in the article was not based on a study that was implemented (n = 1), the article was a review of literature (n = 1), the study was focused on trainee development from a technical perspective (n = 1) (see Fig. 1).
Fig. 1.
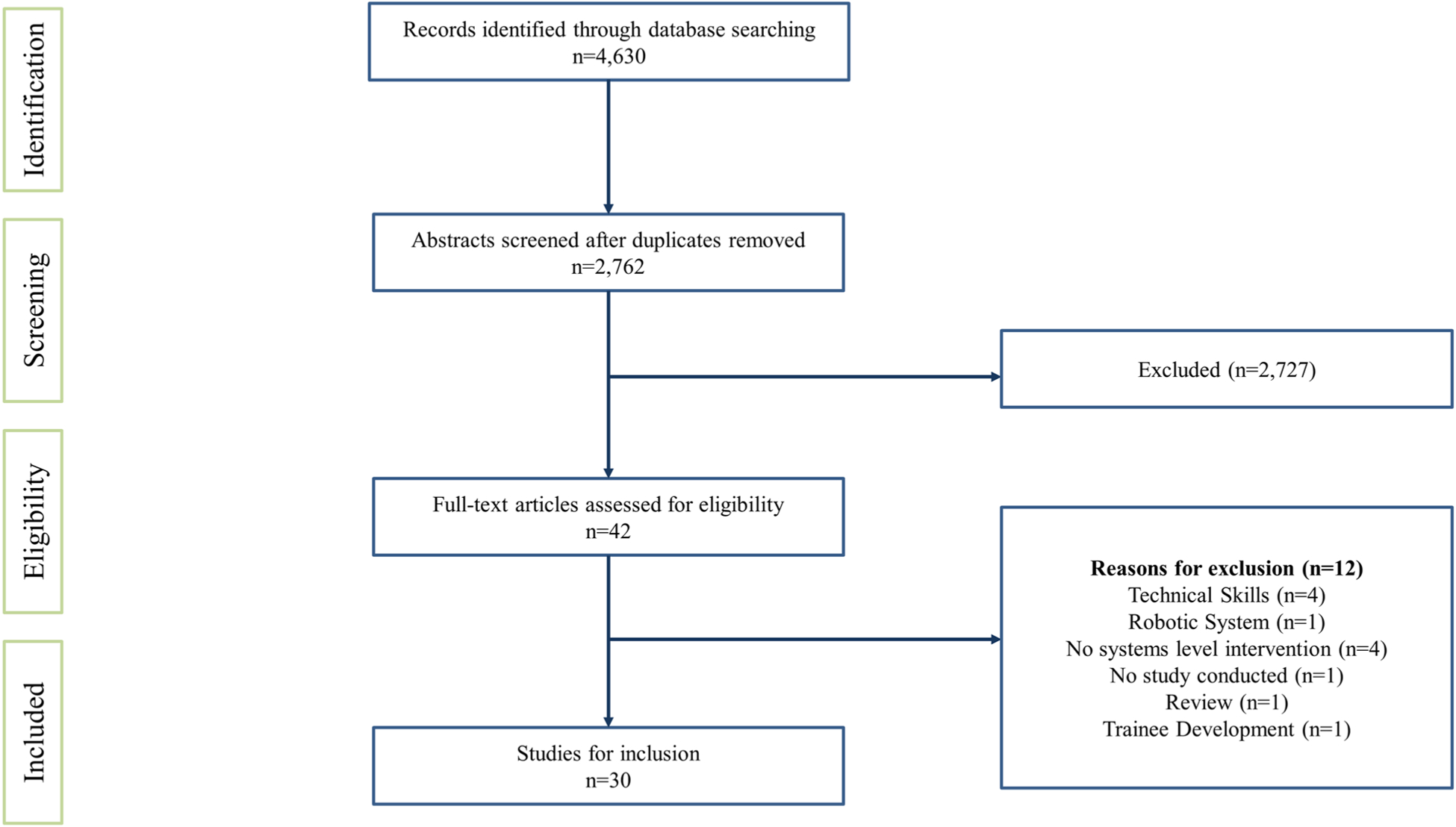
PRISMA Diagram of included studies demonstrating the number of records identified during the database search and abstracts excluded and full-text articles that were included and excluded [30]
Of the articles that were found to discuss various aspects of RAS, 30 articles discussed solutions to existing system-level issues in RAS and were included for analysis; 23 articles discussed proposed interventions based on the issues identified in RAS, and the remaining seven studies implemented interventions and discussed the impact of those interventions.
Studies included in this review were published between 2013 and 2020, with a majority being published during 2016 (n = 7, 23%) and 2018 (n = 6, 20%). Most studies were conducted in the United States (n = 19, 63.33%) followed by the United Kingdom (n = 2, 6.67%). The remaining studies were published in Germany (n = 2, 6.67%), Italy (n = 2, 6.67%), Netherlands (n = 2, 6.67%), New Zealand (n = 1, 3.33%), Norway (n = 1, 3.33%), and Sweden (n = 1, 3.33%).
The mechanism of studies included in this systematic review were included in multiple categories and consisted of observational approaches, which included video, audio, and live observation (n = 20, 66.67%), surveys (n = 11, 36.67%), focus groups or interviews (n = 5, 16.67%), and tool/intervention proposal (n = 4, 13.33%).
The most common work-system problems addressed with proposed, implemented and evaluated interventions were flow disruptions (n = 9, 30%) followed by ergonomic challenges for the surgeon/first assistant (n = 5, 16.70%), challenges with communication (n = 5, 16.70%), safety and efficiency (n = 4, 13.33%), and “non-technical skills” overall (n = 3, 10%). The remaining studies assessed teamwork (n = 2, 6.70%), integration of a robotic system (n = 1, 3.30%) from the perspective of team coordination and operating room environment, and team preparedness (n = 1, 3.30%). Interventions included checklists, team training or methods to improve team coordination, or the redesign of workspace to improve team efficiency in the operating room (see Table 1).
Each article was categorized into one of two groups: (1) articles that suggested an intervention based on the results of a study examining systems-level issues in RAS (n = 23, 76.67%); or (2) articles that discussed the development, implementation and evaluation of an intervention aimed to address systems-level issues in RAS (n = 7, 23.33%). Following the categorization of intervention implementation, the studies were further broken down by the type of intervention discussed. Keeping in mind that some studies evaluated more than one system-level issue, some interventions were included in multiple categories (see Table 1). The categories included training (n = 9, 30%), adjustments to the operating room environment (n = 6, 20%), checklists to improve workflow, preparation, or safety (n = 5, 16.67%), teamwork to improve coordination among operating room staff (n = 5, 16.67%) communication improvement (n = 4, 13.33%), ergonomics (n = 2, 6.67%), use of technology (n = 2, 6.67%), implementation of guidelines (n = 1, 3.33%), optimization of workflow to improve efficiency (n = 1, 3.33%), and team briefings (n = 1, 3.33%).
More specifically, the seven articles that discussed the development, implementation and evaluation of an intervention included checklists (n = 2, 28.57%), ergonomic training (n = 2, 28.57%), robotic to open conversion training (n = 1, 14.29%), development of guidelines for robotic to open conversion (n = 1, 14.29%), and the implementation and evaluation of new technology (n = 1, 14.29%). Interventions were evaluated in both training environments (n = 4, 57.14%) and in operating rooms during real procedures (n = 3, 42.86%). Multiple outcome measures were used to determine the success of the intervention which included surveys (n = 3, 42.86%), evaluation of errors and time (n = 2, 28.57%), focus groups (n = 1, 14.29%), and hospital readmissions over a 30-day period (n = 1, 14.29%). All seven interventions that were implemented were determined to be successful such that the interventions decreased ergonomic strain (n = 2, 28.57%) or improved non-technical skills such as communication, teamwork, workflow, and patient safety. Although the interventions were impactful in improving issues faced in the operating room during RAS, six of the studies reported limitations of the intervention related to the method chosen to test or device chosen to use for the intervention (e.g., simulation, headsets, etc.) (n = 3, 42.86%), reluctancy of staff to adopt the intervention (n = 1, 14.29%), or the subjective nature of responses or assessment of ergonomic strain (n = 2, 28.57%) (see Table 2).
Table 2.
Implemented and evaluated interventions
| Reference | Intervention category | Intervention details | Intervention implementation | Target team members | Outcome measures | Outcome of intervention | Intervention challenges |
|---|---|---|---|---|---|---|---|
| Franasiak [41] | Training | Training on ergonomic modifications to the console workstation and posture | Training implemented over 6 months | Surgeons (n = 32) | Ergonomic strain (measured pre/post intervention)—evaluated using Nordic Musculoskeletal Questionnaire (NMQ) | All surgeons found training to be helpful, and 88% (28/32) changed their practice as a result of the training. Of 19 surgeons who reported strain in the original survey, 14 (74%) noted a decrease in strain after training | Reports of strain are subjective |
| Jing [33] | Checklist | Implementation of a checklist to improve pre-procedure preparation for robotic-assisted prostatectomies and improve safety and efficiency | Use of checklist in 22 robotic-assisted radical prostatectomy procedures over a two-month trial period | OR staff | Focus group feedback (after trial period) | OR staff felt more confident, felt more aware of what was needed when setting up and reported that the surgery was smoother and that there were fewer interruptions because participants were less likely to leave the OR to retrieve a surgical item | n/a |
| McCarroll [34] | Checklist | Implementation of a computerized checklist for robotic-assisted surgery. Developed based of the World Health Organization’s Safe Surgery Saves Lives Checklist | Use of a checklist with 32 OR staff during simulation. The later computerized and implemented in OR for use during robotic-assisted gynecologic procedures over the course of 6 months | OR staff | Number of readmissions that occurred 30-days post surgery (measured pre/post-checklist intervention) | 30 day readmissions pre-checklist (12) and post-checklist (5) were significantly reduced (p = 0.02) | OR staff members were not as motivated to use the checklist if a specific nurse was not present, potentially resulting in a pseudo-Hawthorne effect |
| Tsafrir [52] | Technology | Wireless audio headsets in a robotic operating room to improve the quality of communication | 69 gynecologic and urologic cases conducted with headsets were observed and compared to 68 without | OR staff (n = 148) | A 14-point questionnaire was administered at the conclusion of each case to evaluate communication, performance, teamwork, and mental load (pre/post technology implementation) | Self-reported communication quality was better in cases where headsets were used (113.0 ± 1.6 vs. 101.4 ± 1.6; P < 0.001). Use of headsets reduced percentage of time with a noise level above 70 dB at the console (8.2% ± 0.6 vs. 5.3% ± 0.6, P < 0.001) | Ambient noise levels are not eliminated because the headset covers one ear |
| Van’t Hullenaar [54] | Training | Development of an ergonomic training program to provide instructions for ergonomic setup of the robotic console | Those in an intervention group received a short-written guide on correct ergonomic adjustment of the console and seat, an explanation on correct usage of the clutch controls, verbal coaching regarding posture and correct usage of the clutch | Surgical interns and residents | The Rapid Upper Limb Assessment (RULA) to evaluate ergonomic positioning via video recordings. Local Experienced Discomfort Scale and, NASA Task-Load Index (NASA-TLX) were evaluated (in control and intervention group) | Significantly lower economy of motion (EOM) was found in intervention group, P = 0.013. The overall RULA score for the left (P < 0.001) and right (P < 0.001) body halfs were significantly better in the intervention group. Participants did not report higher stress levels or muscle strain, as measured by the TED and NASA-TLX scores | Optimal interpretation and accuracy of the ergonomic setup are not guaranteed |
| Zattoni [57]a | Guidelines | Development of guidelines for conversion from robotic-assisted radical prostatectomy to open radical prostatectomy | 20 simulations were performed over the course of seven months in a surgical theater for one surgical team to train on open conversions. For each simulation, the team was provided with a conversion protocol to follow for the conversion to open. Each simulation was timed (from the start of the conversion to skin incision) and video recorded. For each simulation, four main strategies were implemented to reduce errors: improving leadership, clearly defining roles, improving knowledge base, and surgical room reorganization. Guidelines were posted in the operating room after the training | Surgeons, Anesthesiologists, and Nurses | Time to conversion, number of errors, problems in the areas of environment, leadership,task sequence and allocation, planning, communication, training, and checklists were assessed prior to the implementation of the training and then reassessed after the training to identify improvement | The average conversion time was 130.9 (interquartile range [IQR] 90–201) seconds. Frequencies of the observed errors were as follows: lack of task sequence (70%), errors in robot movements (50%), loss of sterility (50%), space conflict (40%), communication errors (25%), lack of leadership (25%), and accidental fall of surgical devices (25%). By the last simulation, conversions were performed without errors and using 55.2% less time compared with initial simulations | The guidelines were developed for a specific type of surgery and may not account for other surgical complications and patient factors |
| Zattoni [58]a | Training | Evaluation of a standardized training and institutional checklist on improving teamwork during complications requiring open conversion from robotic-assisted partial nephrectomy | 20 emergencies were simulated: group 1 performed simulations followed by a 4-h theoretical training; group 2 underwent 4-h training first and then performed simulations | OR staff | Conversion time number of errors, problems experienced during conversions in the areas of environment, leadership, task sequence and allocation, planning, communication, training, and checklists were assessed prior to the implementation of the training and after the training to identify improvement | Group 1 showed a higher time to conversion (TC) than group 2 (116.5 vs 86.5 s, P = 0.0.53). TC shows a progressive decline for both groups as the number of simulations increases (group 1, R2 = 0.7 and group 2,R2 = 0.61), but it remains higher for group 1. Lack of task sequence and accidental falls or loss of sterility were higher in group 1 | The simulated environment produces shorter conversion times and may not accurately represent real-life experiences during robotic-assisted surgery |
Interventions were analyzed using the same method
Discussion
The 30 studies that met inclusion criteria support the notion that introducing RAS technology into the operating room presents challenges that force new adaptations to existing routines and processes. Rather than solely focusing on surgical techniques, implementing systems-level solutions can make a positive impact on team dynamics, communication, preparedness, patient recovery and safety. However, many risks and issues with RAS remain unaddressed systematically, evidentially, and in everyday practice. Multiple commentators have observed that the spread of surgical innovation often precedes systems-level safety analysis [5, 14]. RAS is no exception. As robotic systems improve and RAS becomes more popular, it is important to address the current issues that exist within RAS and develop solutions to overcome those barriers.
All 30 studies identified systems-level issues associated with the integration of RAS, and a majority (76.67%) proposed solutions, but did not implement or evaluate the impact. Ahmad et al. [9] explored OR setup by tracking movements of OR staff and argued that approximately 50% of all movements could have been avoided if the OR setup was designed to cater toward the needs and tasks of OR team members. In another example Allers et al. [38] studied flow disruptions in ten robotic-assisted prostatectomy procedures and proposed that improved preparation could aid in decreasing the amount of disruptions that occur during a single procedure and in turn improve efficiency and safety. Similarly, Catchpole et al. [6] examined flow disruptions in RAS in urology, gynecology, and cardiac surgery and suggested that OR staff performance, efficiency, and operative duration could be improved through training and effective communication and coordination among OR staff members. Craven et al. [40] investigated ergonomics of the robotic console through the evaluation of strain experienced by the operating surgeon in gynecologic oncology and suggested that ergonomic training could educate surgeons on proper ergonomic practices. While each of these studies helped to paint a clearer picture of how work is done in the RAS system, the proposed interventions served only as suggestions and were neither investigated nor evaluated.
A much smaller proportion of studies (23.33%) actually implemented and evaluated interventions. A 2016 New Zealand study introduced a surgical checklist for radical prostatectomies [33]. The checklist was used for two months; its use resulted in improved efficiency, increased confidence among OR staff when assisting in a RALRP procedure, and a positive change in teamwork between OR staff [33]. More specifically, through focus groups, it was reported that the checklist was helpful during setup prior to the prostatectomy procedure, allowed the surgical teams to be more prepared for the procedure, and improved teamwork and efficiency while decreasing workflow interruptions [33]. McCarroll and colleagues (2015) also evaluated the efficacy of a checklist in RAS. Here authors investigated the use of a Robotic Operating Room Computerized Checklist during robotic-assisted gynecologic surgeries to reduce readmissions post surgery. The checklist was developed specifically for RAS and included items such as ensuring that the robotic instruments were properly inspected, the surgical team had the opportunity to introduce themselves to the rest of the team prior to performing the initial incision, and equipment issues were addressed before the patient was wheeled out. The implementation of the checklist reduced the number of readmissions without affecting operating times [34].
A lack of evidence-based approaches, and more importantly, standardized interventions to address the challenges in RAS, means that some organizations (or units within them) have developed techniques to prepare their staff for the introduction of this technology, while others have not. Ongoing observational work would suggest that team members adapt to the unique challenges presented by RAS [7]. Consequently, some teams, and some hospital systems, understand and address risks better than others. One criticism of healthcare solutions is that they are human-focused (e.g., encouraging individuals to “try harder”, “work faster”, or “figure it out”) rather than being oriented towards embedding behaviors within the wider socio-technical system. Consequently, there is a need to change the way stakeholders think about issues in RAS. This includes developing interventions that include multiple solutions, from developing checklists to rearranging operating room layout, and evaluating the effects on individuals (teamwork, workload), processes (disruptions, duration), and outcomes (length of stay, blood loss, adverse events).
Though clinical evaluation usually focuses on patient outcomes, this assumes a linear and deterministic relationship. Meaningful process evaluations may be more important for the evaluation of human factors interventions than the outcomes, which do not afford the ability to diagnose and mitigate breakdowns, especially with respect to non-technical skills like teamwork [35]. Furthermore, while individual interventions may have some value within a complex system, a combination of interventions may amplify the effects of a more limited approach. For example, a surgical safety checklist might also benefit from improved teamwork training [36] which in turn might benefit from attention to the OR layout and design of the space. While there has been a recognition of a range of issues associated with RAS, and some suggested interventions, the evaluation of these effects has been less rigorous or absent. A better clinical evidence-based approach would improve the implementation of human factors methods for improving RAS safety and efficiency.
Limitations
This review was focused on identifying interventions developed to address system-level challenges in RAS and excluded studies that failed to suggest or implement interventions. As a result of exclusion criteria, we may not have documented additional work-system challenges in RAS that may have more clearly emphasized the gap between identified challenges and proposed solutions. In other words, this gap may be wider than what was captured in the current review. Moreover, 20 studies involved observational methods for assessing challenges and interventions and may be susceptible to detection bias. Complementary approaches such as Failure Modes and Effects Analysis might be less susceptible to bias, even though such approaches may oversimplify other aspects of the task. Next, by limiting the review to only peer-reviewed published articles, information on suggested or implemented interventions that exist in the gray literature (i.e., literature published in a non-traditional way), conference proceedings, or dissertations may have been missed. In addition, given that work-system breakdowns and interventions encompass a wide range of terms (e.g., teamwork, tools and technology, tasks, workplace environment), some relevant studies may not have been captured, therefore limiting our findings.
Conclusions
The aim of this study was to develop a better understanding of the interventions developed to address work-system challenges associated with RAS. Despite decades of use, interventions to address frequently identified side effects of RAS implementation such as task complexity, ineffective communication, and workspace limitations have only been proposed in the last several years. Moreover, only a handful of these suggested interventions have been implemented and evaluated. The introduction of complex technology (e.g., a surgical robot) to the surgical system drastically changes the way in which work is traditionally done, and while interventions may help teams adapt to new processes, we must bear in mind that the processes we are trying to improve must be studied and addressed systematically. Future work should focus on identifying problems and implementing systems-level interventions aimed at improving OR efficiency during RAS such that disruptions to workflow are minimized, while outcomes related to safety, teamwork, ergonomic practices, and other non-technical skills are improved.
Funding
This project was funded under Grant Number HS026491-01 (PIs Catchpole, Anger, Cohen) from the Agency for Healthcare Research and Quality (AHRQ), U.S. Department of Health and Human Services (HHS). The authors are solely responsible for this document’s contents, findings, and conclusions, which do not necessarily represent the views of AHRQ. Readers should not interpret any statement in this report as an official position of AHRQ or of HHS. None of the authors have any affiliation or financial involvement that conflicts with the material presented in this report.
Disclosures
Falisha Kanji, Dr. Ken Catchpole, Eunice Choi, Dr. Myrtede Alfred, Kate Cohen, Dr. Daniel Shouhed, Dr. Jennifer Anger, and Dr. Tara Cohen report grants from the Agency for Healthcare Research and Quality (AHRQ), U.S. Department of Health and Human Services (HHS), grant number HS026491-01. Furthermore, Dr. Daniel Shouhed discloses an educational honorarium from Intuitive Surgical and Dr. Jennifer Anger discloses an Advisory Board Membership for Axonics.
References
- 1.Barbash GI, Glied SA (2010) New technology and health care costs—the case of robot-assisted surgery. N Engl J Med 363:701–704 [DOI] [PubMed] [Google Scholar]
- 2.Gupta P, Schomburg J, Krishna S, Adejoro O, Wang Q, Marsh B, Nguyen A, Genere JR, Self P, Lund E (2017) Development of a classification scheme for examining adverse events associated with medical devices, specifically the DaVinci surgical system as reported in the FDA MAUDE database. J Endourol 31:27–31 [DOI] [PubMed] [Google Scholar]
- 3.Anger JT, Mueller ER, Tarnay C, Smith B, Stroupe K, Rosenman A, Brubaker L, Bresee C, Kenton K (2014) Robotic compared with laparoscopic sacrocolpopexy: a randomized controlled trial. Obstet Gynecol 123:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sussman RD, Peyronnet B, Brucker BM (2019) The current state and the future of robotic surgery in female pelvic medicine and reconstructive surgery. Turk J Urol 45:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parsons JK, Messer K, Palazzi K, Stroup SP, Chang D (2014) Diffusion of surgical innovations, patient safety, and minimally invasive radical prostatectomy. JAMA Surg 149:845–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catchpole K, Perkins C, Bresee C, Solnik MJ, Sherman B, Fritch J, Gross B, Jagannathan S, Hakami-Majd N, Avenido R, Anger JT (2016) Safety, efficiency and learning curves in robotic surgery: a human factors analysis. Surg Endosc 30:3749–3761 [DOI] [PubMed] [Google Scholar]
- 7.Catchpole KR, Hallett E, Curtis S, Mirchi T, Souders CP, Anger JT (2018) Diagnosing barriers to safety and efficiency in robotic surgery. Ergonomics 61:26–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jain M, Fry BT, Hess LW, Anger JT, Gewertz BL, Catchpole K (2016) Barriers to efficiency in robotic surgery: the resident effect. J Surg Res 205:296–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmad N, Hussein AA, Cavuoto L, Sharif M, Allers JC, Hinata N, Ahmad B, Kozlowski JG, Hashmi Z, Bisantz A, Guru KA (2016) Ambulatory movements, team dynamics and interactions during robot-assisted surgery. BJU Int 118(1):132–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tiferes J, Hussein AA, Bisantz A, Kozlowski JD, Sharif MA, Winder NM, Ahmad N, Allers J, Cavuoto L, Guru KA (2016) The loud surgeon behind the console: understanding team activities during robot-assisted surgery. J Surg Educ 73:504–512 [DOI] [PubMed] [Google Scholar]
- 11.Yu D, Dural C, Morrow MM, Yang L, Collins JW, Hallbeck S, Kjellman M, Forsman M (2017) Intraoperative workload in robotic surgery assessed by wearable motion tracking sensors and questionnaires. Surg Endosc 31:877–886 [DOI] [PubMed] [Google Scholar]
- 12.Randell R, Honey S, Alvarado N, Pearman A, Greenhalgh J, Long A, Gardner P, Gill A, Jayne D, Dowding D (2016) Embedding robotic surgery into routine practice and impacts on communication and decision making: a review of the experience of surgical teams. Cogn Technol Work 18:423–437 [Google Scholar]
- 13.Catchpole K, Bisantz A, Hallbeck S, Weigl M, Randell R, Kos-sack M, Anger J (2018) Human factors in robotic assisted surgery: lessons from studies ‘in the wild.’ Appl Ergon 78:270–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Catchpole K, Perkins C, Bresee C, Solnik MJ, Sherman B, Fritch J, Gross B, Jagannathan S, Hakami-Majd N, Avenido R (2016) Safety, efficiency and learning curves in robotic surgery: a human factors analysis. Surg Endosc 30:3749–3761 [DOI] [PubMed] [Google Scholar]
- 15.Lai F, Entin E (2005) Robotic surgery and the operating room team. SAGE, Los Angeles, pp 1070–1073 [Google Scholar]
- 16.Lingard L, Regehr G, Orser B, Reznick R, Baker GR, Doran D, Espin S, Bohnen J, Whyte S (2008) Evaluation of a preoperative checklist and team briefing among surgeons, nurses, and anesthesiologists to reduce failures in communication. Arch Surg 143:12–17 [DOI] [PubMed] [Google Scholar]
- 17.Greenberg CC, Regenbogen SE, Studdert DM, Lipsitz SR, Rogers SO, Zinner MJ, Gawande AA (2007) Patterns of communication breakdowns resulting in injury to surgical patients. J Am Coll Surg 204:533–540 [DOI] [PubMed] [Google Scholar]
- 18.Rogers SO, Gawande AA, Kwaan M, Puopolo AL, Yoon C, Brennan TA, Studdert DM (2006) Analysis of surgical errors in closed malpractice claims at 4 liability insurers. Surgery 140:25–33 [DOI] [PubMed] [Google Scholar]
- 19.Bismark MM, Brennan TA, Paterson RJ, Davis PB, Studdert DM (2006) Relationship between complaints and quality of care in New Zealand: a descriptive analysis of complainants and non-complainants following adverse events. BMJ Qual Saf 15:17–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaye AD, Vadivelu N, Ahuja N, Mitra S, Silasi D, Urman RD (2013) Anesthetic considerations in robotic-assisted gynecologic surgery. Ochsner J 13:517–524 [PMC free article] [PubMed] [Google Scholar]
- 21.Campos J, Ueda K (2014) Update on anesthetic complications of robotic thoracic surgery. Miner Anestesiol 80:83–88 [PubMed] [Google Scholar]
- 22.Young RS, O’Regan DJ (2010) Cardiac surgical theatre traffic: time for traffic calming measures? Interact Cardiovasc Thorac Surg 10:526–529 [DOI] [PubMed] [Google Scholar]
- 23.Ofek E, Pizov R, Bitterman N (2006) From a radial operating theatre to a self-contained operating table. Anaesthesia 61:548–552 [DOI] [PubMed] [Google Scholar]
- 24.Brogmus G, Leone W, Butler L, Hernandez E (2007) Best practices in OR suite layout and equipment choices to reduce slips, trips, and falls. AORN J 86:384–394; quiz 395–388 [DOI] [PubMed] [Google Scholar]
- 25.Ahmad N, Hussein AA, Cavuoto L, Sharif M, Allers JC, Hinata N, Ahmad B, Kozlowski JD, Hashmi Z, Bisantz A (2016) Ambulatory movements, team dynamics and interactions during robot-assisted surgery. BJU Int 118:132–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yule S, Flin R, Maran N, Youngson G, Mitchell A, Rowley D, Paterson-Brown S (2008) Debriefing surgeons on non-technical skills (NOTSS). Cogn Technol Work 10:265–274 [Google Scholar]
- 27.Russ S, Rout S, Sevdalis N, Moorthy K, Darzi A, Vincent C (2013) Do safety checklists improve teamwork and communication in the operating room? A systematic review. Ann Surg 258:856–871 [DOI] [PubMed] [Google Scholar]
- 28.Hales BM, Pronovost PJ (2006) The checklist—a tool for error management and performance improvement. J Crit Care 21:231–235 [DOI] [PubMed] [Google Scholar]
- 29.Collins JW, Dell’Oglio P, Hung AJ, Brook NR (2018) The importance of technical and non-technical skills in robotic surgery training. Eur Urol Focus 4(5):674–676 [DOI] [PubMed] [Google Scholar]
- 30.Moher D, Liberati A, Tetzlaff J (2009) Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghezzi TL, Corleta OC (2016) 30 years of robotic surgery. World J Surg 40:2550–2557 [DOI] [PubMed] [Google Scholar]
- 32.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A (2016) Rayyan—a web and mobile app for systematic reviews. Syst Rev 5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jing J, Honey MLL (2016) Using a checklist in robotic-assisted laparoscopic radical prostatectomy procedures. AORN J 104:145–152 [DOI] [PubMed] [Google Scholar]
- 34.McCarroll ML, Zullo MD, Roulette GD, Mendise TM, Ferris E, Zolton J, Andrews SJ, von Gruenigen VE (2015) Development and implementation results of an interactive computerized surgical checklist for robotic-assisted gynecologic surgery. J Robot Surg 9:11–18 [DOI] [PubMed] [Google Scholar]
- 35.Salas E, Rosen MA, Weaver SJ, Held JD, Weissmuller JJ (2009) Guidelines for performance measurement in simulation-based training. Ergon Des 17:12–18 [Google Scholar]
- 36.Catchpole K, Russ S (2015) The problem with checklists. BMJ Qual Saf 24:545–549 [DOI] [PubMed] [Google Scholar]
- 37.Ahmed K, Khan N, Khan MS, Dasgupta P (2013) Development and content validation of a surgical safety checklist for operating theatres that use robotic technology. BJU Int 111:1161–1174 [DOI] [PubMed] [Google Scholar]
- 38.Allers JC, Hussein AA, Ahmad N, Cavuoto L, Wing JF, Hayes RM, Hinata N, Bisantz AM, Guru KA (2016) Evaluation and impact of workflow interruptions during robot-assisted surgery. Urology 92:33–37 [DOI] [PubMed] [Google Scholar]
- 39.Craven R, Franasiak J, Mosaly P, Gehrig PA (2013) Ergonomic deficits in robotic gynecologic oncology surgery: a need for intervention. J Minimally Invasive Gynecol 20:648–655 [DOI] [PubMed] [Google Scholar]
- 40.Dru CJ, Anger JT, Souders CP, Bresee C, Weigl M, Hallett E, Catchpole K (2017) Surgical flow disruptions during robotic-assisted radical prostatectomy. Can J Urol 24:8814–8821 [PMC free article] [PubMed] [Google Scholar]
- 41.Franasiak J, Craven R, Mosaly P, Gehrig PA (2014) Feasibility and acceptance of a robotic surgery ergonomic training program. J Soc Laparoendosc Surg 18(4):e2014.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Myklebust MV, Storheim H, Hartvik M, Dysvik E (2020) Anesthesia professionals’ perspectives of teamwork during robotic-assisted surgery. AORN J 111:87–96 [DOI] [PubMed] [Google Scholar]
- 43.Raheem S, Ahmed YE, Hussein AA, Johnson A, Cavuoto L, May P, Cole A, Wang D, Ahmad B, Hasasneh A (2018) Variability and interpretation of communication taxonomy during robot-assisted surgery: do we all speak the same language? BJU Int 122:99–105 [DOI] [PubMed] [Google Scholar]
- 44.Randell R, Honey S, Alvarado N, Greenhalgh J, Hindmarsh J, Pearman A, Jayne D, Gardner P, Gill A, Kotze A (2019) Factors supporting and constraining the implementation of robot-assisted surgery: a realist interview study. BMJ Open 9:e028635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schiff L, Tsafrir Z, Aoun J, Taylor A, Theoharis E, Eisenstein D (2016) Quality of communication in robotic surgery and surgical outcomes. J Soc Laparoendosc Surg 20:e2016.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schuessler Z, Scott Stiles A, Mancuso P (2020) Perceptions and experiences of perioperative nurses and nurse anaesthetists in robotic-assisted surgery. J Clin Nurs 29:60–74 [DOI] [PubMed] [Google Scholar]
- 47.Sexton K, Johnson A, Gotsch A, Hussein AA, Cavuoto L, Guru KA (2018) Anticipation, teamwork and cognitive load: chasing efficiency during robot-assisted surgery. BMJ Qual Saf 27:148–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song JB, Vemana G, Mobley JM, Bhayani SB (2013) The second “time-out”: a surgical safety checklist for lengthy robotic surgeries. Patient Saf Surg 7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Souders CP, Catchpole K, Hannemann A, Lyon R, Eilber KS, Bresee C, Cohen T, Weigl M, Anger JT (2019) Flow disruptions in robotic-assisted abdominal sacrocolpopexy: does robotic surgery introduce unforeseen challenges for gynecologic surgeons? Int Urogynecol J 30:2177–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tiferes J, Hussein AA, Bisantz A, Higginbotham DJ, Sharif M, Kozlowski J, Ahmad B, O’Hara R, Wawrzyniak N, Guru K (2019) Are gestures worth a thousand words? Verbal and nonverbal communication during robot-assisted surgery. Appl Ergon 78:251–262 [DOI] [PubMed] [Google Scholar]
- 51.Tsafrir Z, Janosek-Albright K, Aoun J, Diaz-Insua M, Abd-El-Barr A-E-R, Schiff L, Talukdar S, Menon M, Munkarah A, Theoharis E (2020) The impact of a wireless audio system on communication in robotic-assisted laparoscopic surgery: a prospective controlled trial. PLoS ONE 15:e0220214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van’t Hullenaar CDP, Bos P, Broeders IAMJ (2019) Ergonomic assessment of the first assistant during robot-assisted surgery. J Robot Surg 13:283–288 [DOI] [PubMed] [Google Scholar]
- 53.Van’t Hullenaar CDP, Mertens AC, Ruurda JP, Broeders I (2018) Validation of ergonomic instructions in robot-assisted surgery simulator training. Surg Endosc 32:2533–2540 [DOI] [PubMed] [Google Scholar]
- 54.Wastler KE (2015) Robotic surgical and anesthesia communication tool. J Robot Surg 9:97–98 [DOI] [PubMed] [Google Scholar]
- 55.Weber J, Catchpole K, Becker AJ, Schlenker B, Weigl M (2018) Effects of flow disruptions on mental workload and surgical performance in robotic-assisted surgery. World J Surg 42:3599–3607 [DOI] [PubMed] [Google Scholar]
- 56.Weigl M, Weber J, Hallett E, Pfandler M, Schlenker B, Becker A, Catchpole K (2018) Associations of intraoperative flow disruptions and operating room teamwork during robotic-assisted radical prostatectomy. Urology 114:105–113 [DOI] [PubMed] [Google Scholar]
- 57.Zattoni F, Guttilla A, Crestani A, De Gobbi A, Cattaneo F, Mos-chini M, Vianello F, Valotto C, Dal Moro F, Zattoni F (2015) The value of open conversion simulations during robot-assisted radical prostatectomy: implications for robotic training curricula. J Endourol 29:1282–1288 [DOI] [PubMed] [Google Scholar]
- 58.Zattoni F, Morlacco A, Cattaneo F, Soligo M, Meggiato L, Modo-nutti D, Valotto C, Dal Moro F, Zattoni F (2017) Development of a surgical safety training program and checklist for conversion during robotic partial nephrectomies. Urology 109:38–43 [DOI] [PubMed] [Google Scholar]


