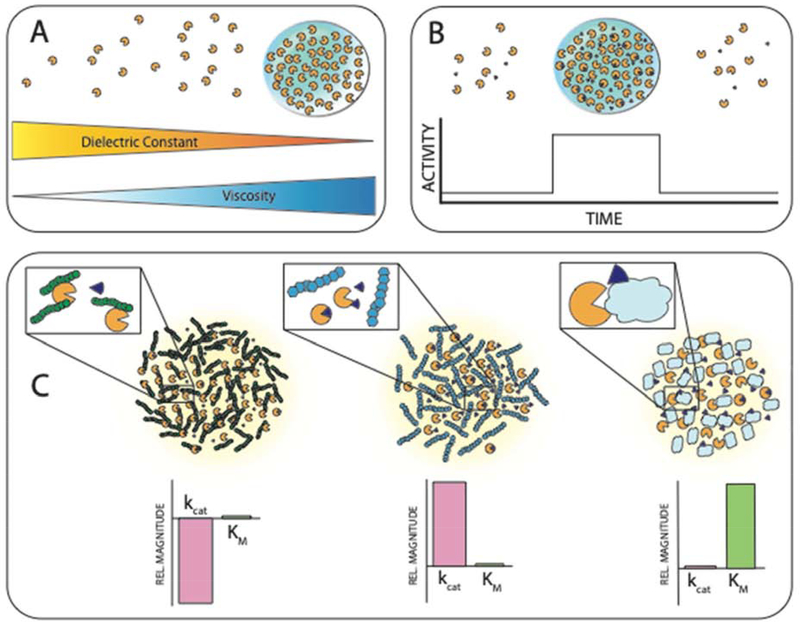
Figure 2: Biophysical and biochemical effects of phase separation can function to regulate enzyme activity.
A. LLPS results in a unique local environment that differs from the surrounding dilute phase. The solvent properties of condensates can resemble organic solvents, allowing tuning of reaction conditions by LLPS. The resulting increase in viscosity has been shown to reduce enzyme activity due to reduced mobility of reagents. This implies a balance between contrasting effects to realize optimal reaction conditions within condensates [2,22,75,76]. B. The high cooperativity of LLPS means that enzymatic reactions can be switched on or off. By balancing the concentration of enzyme at the threshold of phase separation (likely through translational regulation), activity can be rapidly switched on by a small increase in local concentration, and off again by a subsequent small decrease depending on cellular needs [5,14,50,61]. C. Crowding within a condensate can result in a variety of effects on kinetic activity. Interactions between components in a condensate can result in a reduced rate of product turnover by freezing enzyme dynamics critical to turnover. Non-interacting components can reduce the available space through their excluded volume, concentrating enzymes together with their substrates, resulting in increased turnover. Finally, components which form weak, nonspecific interactions with the substrate and reduce accessibility to the enzyme active site can result in an apparent reduction in enzyme-substrate affinity [74].