Abstract
Background:
Despite the rapid adoption of transcatheter aortic valve replacement (TAVR) since its initial approval in 2011, the frequency and outcomes of surgical explantation of TAVR devices (TAVR-explant) is poorly understood.
Methods:
Patients undergoing TAVR-explant between January 2012–June 2020 at 33 hospitals in Michigan were identified in the Society of Thoracic Surgeons (STS) Database and linked to index TAVR data from the Transcatheter Valve Therapy (TVT) Registry through a statewide quality collaborative. The primary outcome was operative mortality. Indications for TAVR-explant, contraindications to redo TAVR, operative data, and outcomes were collected from STS and TVT databases. Baseline STS Predicted Risk of Mortality (PROM) was compared between index TAVR and TAVR-explant.
Results:
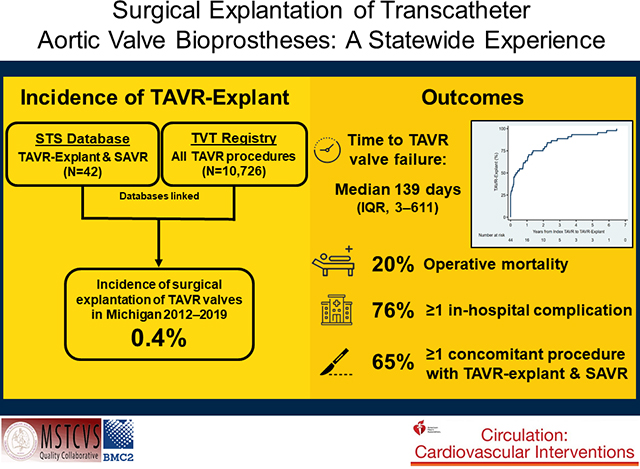
24 surgeons at 12 hospitals performed TAVR-explants in 46 patients (median age 73). The frequency of TAVR-explant was 0.4% and the number of explants increased annually. Median time to TAVR-explant was 139 days and among known device types explanted, most were self-expanding valves (29/41, 71%). Common indications for TAVR-explant were procedure-related failure (35%), paravalvular leak (28%), and need for other cardiac surgery (26%). Contraindications to redo TAVR included need for other cardiac surgery (28%), unsuitable non-coronary anatomy (13%), coronary obstruction (11%), and endocarditis (11%). Overall 65% (30/46) of patients underwent concomitant procedures, including aortic repair/replacement in 33% (n=15), mitral surgery in 22% (n=10), and CABG in 16% (n=7). The median STS-PROM was 4.2% at index TAVR and 9.3% at TAVR-explant (p=0.001). Operative mortality was 20% (9/46) and 76% (35/46) of patients had in-hospital complications. Of patients alive at discharge, 37% (17/37) were discharged home and overall 3-month survival was 73±14%.
Conclusions:
TAVR-explant is rare but increasing, and its clinical impact is substantial. As the utilization of TAVR expands into younger and lower risk patients, providers should consider the potential for future TAVR-explant during selection of an initial valve strategy.
Keywords: transcatheter aortic valve implantation, transcatheter aortic valve, aortic stenosis, aortic valve, aortic valve replacement, Aortic Valve Replacement/Transcather Aortic Valve Implantation, Cardiovascular Surgery, Catheter-Based Coronary and Valvular Interventions, Mortality/Survival, Quality and Outcome
Graphical Abstract
INTRODUCTION
Transcatheter aortic valve replacement (TAVR) is an established alternative to surgical aortic valve replacement (SAVR) for patients with severe symptomatic aortic stenosis, with a growing body of evidence demonstrating the short- and intermediate-term durability of current TAVR devices.1,2 TAVR has rapidly advanced from its original use in patients at prohibitive surgical risk to those at low risk over the past decade.3,4 Since the inception of TAVR, an increasing number of patients are requiring procedures for failed TAVR valves. Initial reports of repeat TAVR for failed TAVR valves (e.g., redo TAVR) define an incidence of 0.33–0.40%1,2 and a large international series recently reported excellent short-term outcomes at 30-days and 1-year for patients undergoing redo TAVR.2
However, these redo TAVRs were performed in select patients with suitable anatomy, while the number of TAVR valves requiring surgical explantation and SAVR (TAVR-explant) due to unsuitable anatomy or other contraindications to redo TAVR were not reported. In addition, long-term outcomes after redo TAVR are unknown and the clinical impact of TAVR-explant to address TAVR valve dysfunction has not been well-described. An analysis of the Society of Thoracic Surgeons (STS) Adult Cardiac Surgery Database (ACSD) described TAVR-explant procedures as rare but morbid compared with similar patients undergoing primary SAVR.5 However, these data only extend to March 2015 and more contemporary single-center6 and STS ACSD7 analyses have shown that the majority of TAVR-explants have occurred more recently. A different national analysis of Medicare beneficiaries found an incidence of 0.2% and comparable 30-day mortality to other series, but ends in 2017 and lacks clinical detail for concomitant surgical procedures, such as aortic repair.8 Furthermore, none of these national analyses include procedural data from the index TAVR.5,7–8 Merging clinical data from the index TAVR and subsequent TAVR-explant is essential to fully characterize the lifetime management of aortic stenosis in these patients.
Therefore, we linked data from the STS ACSD and Transcatheter Valve Therapy (TVT) Registry through a statewide quality collaborative to: 1) define the frequency of TAVR-explant in Michigan and 2) report the indications for and outcomes after TAVR-explant. We hypothesize that the frequency of TAVR-explantation will be comparable to published rates and that older explanted TAVR valves will be associated with more frequent and complex concomitant procedures.
METHODS
Data Sources
Clinical data for surgical TAVR-explant procedures were collected from the STS ACSD through the Michigan Society of Thoracic and Cardiovascular Surgeons Quality Collaborative (MSTCVS-QC), developed in 2001 as a cardiac surgeon-led quality collaborative embedded in the MSTCVS including all 33 non-federal hospitals performing cardiac surgery in Michigan.
Clinical data for TAVR procedures were collected through Michigan TAVR, a collaboration between the MSTCVS and Blue Cross Blue Shield Cardiovascular Consortium (BMC2). The Michigan TAVR Coordinating Center receives quarterly data for Michigan from the STS/American College of Cardiology (ACC) TVT Registry.
This University of Michigan Institutional Review Board deemed this study to be exempt from review (HUM00185363) and the requirement for informed consent was waived. These data cannot be made available due to data use restrictions. Additional details pertaining to analytic methods are available from the corresponding author upon reasonable request.
Patient Population
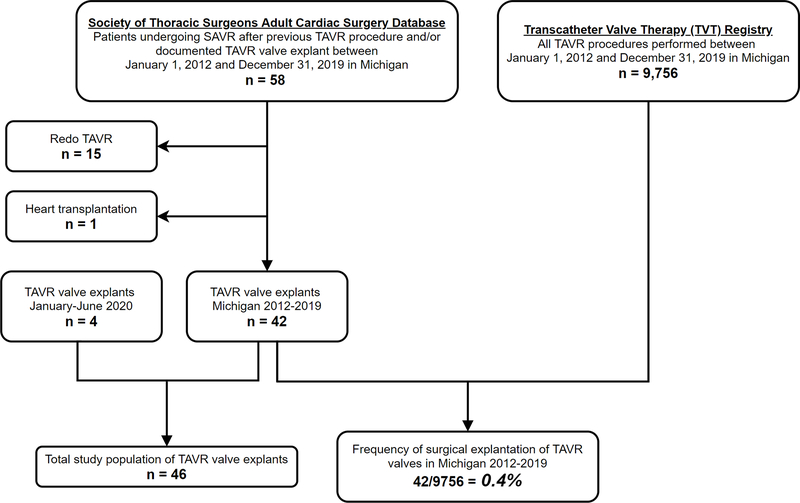
Patients undergoing SAVR and with a documented prior TAVR procedure and/or documented TAVR valve explant between January 1, 2012–December 31, 2019 in Michigan were identified from the STS ACSD [n=58]. From these, 15 patients who underwent redo TAVR and 1 undergoing heart transplantation after prior TAVR were excluded. The total number of TAVRs performed during the same period was collected from the TVT Registry and used as the denominator for frequency. An additional 4 patients undergoing TAVR-explant procedures between January–June 2020 were included to total 46 TAVR-explant patients in the final population (Figure 1).
Figure 1.
Flow diagram of patient population. SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement.
STS dates of birth, sex, height, race/ethnicity, and date of primary TAVR (if known) were used to develop an algorithm to match STS records of TAVR-explant operations to TVT Registry records for index TAVR procedures. In total 91% (42/46) of patients were successfully linked. Reasons for unsuccessful matching may include index TAVRs performed in a different state or enrollment in a trial at the time of index TAVR. Date of primary TAVR was available for 96% (44/46) of patients.
Surgical Explantation of TAVR Bioprostheses
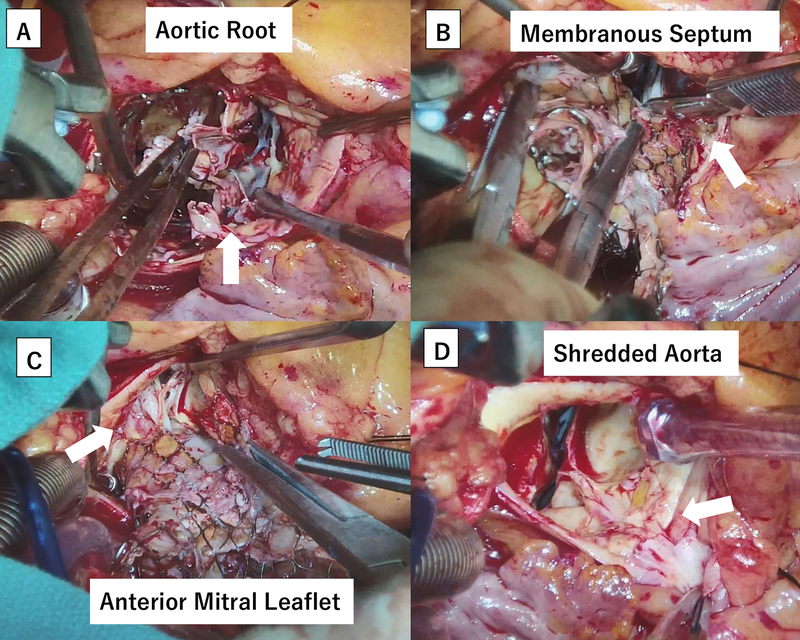
Surgical technique for TAVR-explant procedures was determined according to surgeon preference. Circumferential device neoendotheliazation was often present in older valve explants and careful dissection was required to avoid structural injuries to the aorta, anterior mitral leaflet, and the membranous septum (Figure 2A–D).
Figure 2.
Intraoperative images of a 3-year-old transcatheter aortic valve replacement (TAVR) self-expanding valve explant. (A) Denuded aortic intima due to severe endothelialization. (B) Severely adherent stent cage to the membranous septum. (C) Severely adherent stent cage to the anterior mitral leaflet and chordae tendinae. (D) Disintegrated right coronary sinus after the TAVR valve removal.
Explanted prostheses were either self-expanding (Medtronic Inc, Minneapolis, MN) (n=29, 71%) or balloon-expandable (Edwards Lifesciences, Irvine, CA) (n=12, 29%) devices and unknown in 5 patients. The surgical technique of early (<1 year) and late (>1 year) explantation of both self-expanding and balloon-expandable devices has been described previously.6,9
Definitions and Outcomes
The primary outcome was operative mortality, defined as death during the hospitalization or within 30 days after TAVR-explant. A subgroup analysis included STS predicted risk of mortality (PROM) for isolated SAVR, which was reported at index TAVR in 91% (42/46), TAVR-explant in 72% (33/46), and available at both initial TAVR and TAVR-explant in 67% (31/46).
Secondary outcomes included time to TAVR-explant, index TAVR echocardiographic data, need for concomitant procedures during TAVR-explant, in-hospital complications, discharge location, 30-day readmission, and all-cause mortality. Individual in-hospital complications included permanent stroke, reoperation for bleeding, new renal failure, postoperative atrial fibrillation, and new permanent pacemaker placement. Echocardiographic data before and after index TAVR was collected from the TVT Registry. Late TAVR-explant occurred >1 year after the initial TAVR procedure and early explant occurred <1 year from initial TAVR, as defined previously.2,6
Indications for TAVR-explant were collected from the STS ACSD versions 2.73, 2.81, and 2.9 and could include >1 per patient (eAppendix). Patients with a “need for other cardiac surgery” indication also met ≥1 valve-related indications for TAVR-explant. Indications “failed repair” and “sizing/position issue” as defined in the STS ACSD were combined and categorized as “procedure-related failure,” to encompass devices which failed either during the index TAVR or afterwards for reasons directly related to the procedure. Patient TVT and STS data were used by the authors to determine contraindications to redo TAVR for 74% (34/46) of patients, which also sometimes included >1 per patient.
Statistical Analysis
Normally-distributed continuous variables are expressed as mean ± standard deviation and non-normally distributed variables are expressed as median (interquartile range). Bivariate comparisons utilized paired, two-tailed t-tests for normally-distributed continuous variables, Wilcoxon rank-sum tests for non-normally distributed continuous variables, and Fisher’s exact tests for categorical variables.
A spaghetti plot displayed STS-PROM at initial TAVR and TAVR-explant and medians were compared with the Wilcoxon rank-sum test. Time-to-event survival analyses were performed using the log-rank test and Kaplan-Meier estimates with corresponding 95% confidence intervals (CIs). Two time-to-event analyses were performed: cumulative frequency of TAVR-explant from date of index TAVR with TAVR-explant treated as a failure event and cumulative survival after TAVR-explant.
P-values <0.05 (2-tailed) were considered statistically significant. Analyses were conducted using Stata 16.0 (StataCorp LLC, College Station, TX).
RESULTS
TAVR-Explant Frequency
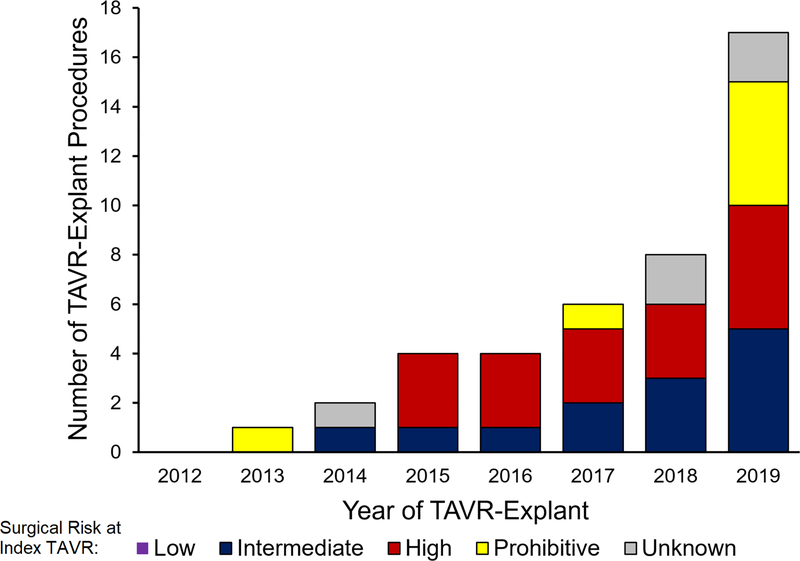
The frequency of TAVR-explant between 2012–2019 was 0.43% (42/9,756), and the number of TAVR-explants increased annually from 1 in 2013 to 17 in 2019 (Figure 3) while the number of TAVR procedures also increased from 141 in 2012 to 2404 in 2019 (eFigure 1). Among 157 cardiac surgeons at 33 hospitals in Michigan, 15% (n=24) of surgeons at 36% (12/42) of hospitals performed ≥1 TAVR-explant (median, 1; range, 1–12 per surgeon).
Figure 3.
Number of TAVR (transcatheter aortic valve replacement)-explants per year. Each year is stratified by patient risk at index TAVR (low, intermediate, high, or prohibitive).
Patient Characteristics
Mean age was 73±8 years and 33% (n=15) were female (Table 1). At the time of TAVR-explant, 50% (n=23) had chronic lung disease, 43% (n=20) a history of cerebrovascular disease, 15% (n=7) prior stroke, and 28% (n=13) a permanent pacemaker. Mean left ventricular ejection fraction was 50±14%, 14% (n=6) of patients had undergone a prior CABG, 11% (n=5) had a porcelain aorta, and 7% (n=3) had a history of mediastinal radiation.
Table 1.
Patient characteristics at time of TAVR (transcatheter aortic valve replacement)-explant. Values are expressed as number (%), mean±standard deviation, or median (interquartile range).
| Characteristic | Overall (n=46) | Early Explant (<1 year, n=28) | Late Explant (>1 year, n=16) | P-value |
|---|---|---|---|---|
| Age, years | 73±8 | 75±8 | 71±8 | 0.15 |
| Female sex | 15(33) | 6(21) | 8(50) | 0.09 |
| Hypertension | 41(89) | 23(82) | 16(100) | 0.14 |
| Diabetes | 16(35) | 8(29) | 7(44) | 0.34 |
| Dialysis | 6(13) | 4(14) | 2(13) | 1.00 |
| Chronic lung disease | 23(50) | 12(43) | 9(56) | 0.53 |
| Cerebrovascular disease | 20(43) | 13(46) | 6(38) | 0.75 |
| Prior stroke | 7(15) | 5(18) | 2(13) | 1.00 |
| Permanent pacemaker | 13(28) | 9(32) | 4(25) | 0.74 |
| Peripheral vascular disease | 9(20) | 6(21) | 3(19) | 1.00 |
| Previous myocardial infarction | 17(37) | 10(36) | 7(44) | 0.75 |
| Body mass index, kg/m2 | 30.9±8.0 | 28.3±8.1 | 34.1±6.3 | 0.018 |
| Left ventricular ejection fraction | 50±14% | 51±15% | 49±13% | 0.69 |
| Porcelain aorta | 5(11) | 2(7) | 3(19) | 0.34 |
| History of mediastinal radiation | 3(7) | 0 | 3(19) | 0.042 |
| Prior CABG | 6(14) | 3(12) | 3(19) | 0.66 |
| NYHA class III/IV (n=39) | 34(87) | 19(83) | 13(93) | 0.63 |
| STS predicted risk of mortality, median (interquartile range) [n=33] | 8.9%(5.4–18.2) | 6.5%(3.8–17.3) | 14.0%(7.3–33.6) | 0.13 |
| Low (<4%) | 6(18) | 5(26) | 1(8) | 0.44 |
| Intermediate (4–8%) | 8(24) | 5(26) | 3(23) | |
| High (>8%) | 19(58) | 9(47) | 9(69) |
CABG, coronary artery bypass grafting; NYHA, New York Heart Association; STS, Society of Thoracic Surgeons.
Eighty-seven percent (34/39) of patients presented with New York Heart Association (NYHA) functional class III/IV heart failure. Among those with available STS-PROM (n=33), 58% (n=19) were at high surgical risk (>8%). The overall median STS-PROM at TAVR-explant was 8.9% (5.4–18.2), which was higher for late versus early explants (14% [7.3–33.6]) vs. 6.5% [3.8–17.3]; p=0.13; Table 1); however, this did not reach statistical significance.
Index TAVR Data
Data from the index TAVR were available for 91% (42/46) patients (eTable 1). Most presented with NYHA III/IV (n=31) symptoms and the 2 most common indications for TAVR were primary aortic stenosis (n=26, 63%) and failed bioprosthetic valve requiring valve-in-valve (VIV) procedures (n=14, 33%). Thirty-three percent of patients (n=14) were classified as intermediate risk for surgery at time of TAVR, 48% (n=20) were high risk, and 19% (n=8) were prohibitive/extreme risk.
Between 2012–2019 in Michigan, 40.3% (3935/9756) of TAVR devices implanted were self-expanding, while 59.2% (5771/9756) were balloon-expandable. Among eventual TAVR-explants, 71% (n=29) had a self-expanding device implanted at the index TAVR, while 29% (n=12) patients received a balloon-expandable device. Due to intra-procedural positioning errors, a second self-expanding TAVR device was implanted in 3 patients during the index TAVR procedure.
On pre-TAVR echocardiogram, 5% (n=2) patients had mitral stenosis (MS), 32% (n=13) had moderate or worse mitral regurgitation (MR), and 23% (n=9) had moderate or worse tricuspid regurgitation (TR). Post-TAVR echocardiogram showed mild paravalvular leak (PVL) in 21% (n=8) of patients and moderate PVL in 15% (n=5), while the rest had none.
Indications for TAVR-Explant and Operative Data
The most common indications for TAVR-explant included procedure-related failure (35%), paravalvular leak (28%), need for other cardiac surgery (26%), and endocarditis (13%) [Figure 4A and eFigures 2–3]. Contraindications to redo TAVR included need for other cardiac surgery (28%), unsuitable non-coronary anatomy (13%), risk of coronary obstruction (11%), endocarditis (11%), and were unknown in 26% (n=12) of patients (Figure 4B). Unsuitable non-coronary anatomy included prior VIV procedures in 4 patients and an oversized annulus perimeter in 2 patients, precluding redo TAVR. In the subgroup analysis of 31 patients with complete STS-PROM scores, the median STS-PROM was significantly higher at TAVR-explant (9.3% [5.6–18.8]) compared with index TAVR (4.2% [2.5–8.9]; p=0.001, Figure 5).
Figure 4.
Procedure indications and contraindications. A) Indications for TAVR-explant and B) Contraindications to redo TAVR. TAVR-explant indications were available for all patients, while contraindications to redo TAVR were determined for 34/46 (74%) patients. Some had more than one indication and/or contraindication. Patients with a need for other cardiac surgery also met ≥1 valve-related indications for TAVR-explant.
Figure 5.
Society of Thoracic Surgeons (STS) predicted risk of mortality (PROM) at index transcatheter aortic valve replacement (TAVR) and TAVR-explant. An STS-PROM was available at both times in 67% (31/46) of patients.
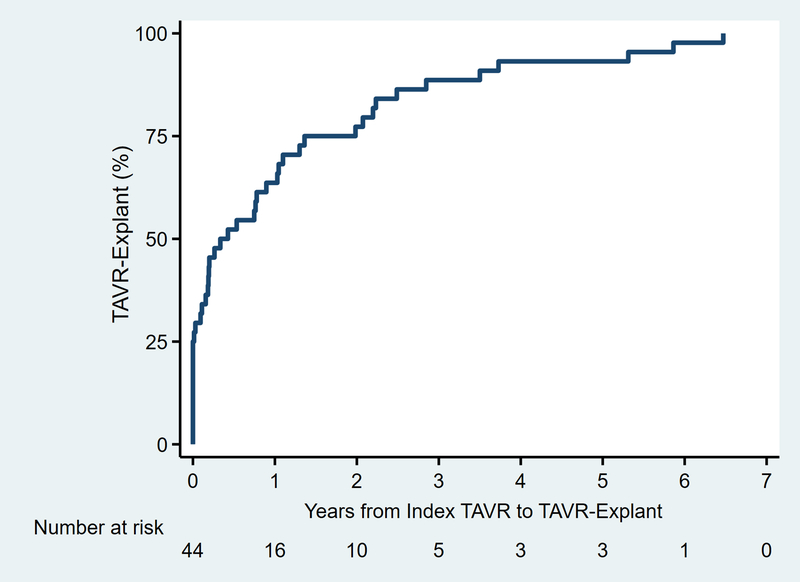
Among patients with known procedure dates (n=44), the median time between TAVR and TAVR-explant was 139 (3–611) days (Figure 6), including 11 patients (25%) who underwent emergent/urgent conversion to TAVR-explant and SAVR on the same day as the index TAVR. All other patients (33/44, 75%) underwent TAVR-explant during a subsequent hospitalization after index TAVR. Sixty-one percent of patients (n=28/46) had undergone at least one previous sternotomy, more frequently among late versus early explants (81% [13/16] vs. 46% [13/28], p=0.030). A higher proportion of late explants underwent elective procedures, while more early explants were emergent (Table 2). Median cardiopulmonary bypass and cross-clamp times were 165 (131–235) and 121 (95–174) minutes, respectively. Seventy-eight percent of patients (n=36) received a stented bioprosthesis, while 4% (n=2) received a stentless bioprosthesis and 7% (n=3) a mechanical valve.
Figure 6.
Time to valve failure from index transcatheter aortic valve replacement (TAVR) to TAVR-explant. Two patients (2/46, 4%) were excluded due to an unknown date of index TAVR.
Table 2.
TAVR (transcatheter aortic valve replacement)-explant operative data. Values are expressed as number (%) or median (interquartile range).
| Characteristic | Overall (n=46) | Early Explant (<1 year, n=28) | Late Explant (>1 year, n=16) | P-value |
|---|---|---|---|---|
| Redo sternotomy | 28(61) | 13(46) | 13(81) | 0.030 |
| Operative status | ||||
| Elective | 20(43) | 9(32) | 10(63) | 0.025 |
| Urgent | 18(39) | 11(39) | 6(38) | |
| Emergent/salvage | 8(17) | 8(29) | 0 | |
| Cardiopulmonary bypass time, min | 165(131–235) | 159(131–234) | 193(131–253) | 0.62 |
| Cross-clamp time, min | 121(95–174) | 120(85–155) | 153(105–184) | 0.20 |
| Circulatory arrest | 5(11) | 5(18) | 0 | 0.14 |
| Explanted device type | ||||
| Balloon-expandable | 12(26) | 9(32) | 3(19) | 0.19 |
| Self-expanding | 29(63) | 16(57) | 13(81) | |
| Unknown | 5(11) | 3(11) | 0 | |
| Explanted device size, mm | 29(26–34)* | 29(26–34) | 27.5(23–31) | 0.17 |
| Explanted device age, days | 139(3–611)* | 37(0–109) | 809(486–1320) | <0.001 |
| Implanted prosthesis | ||||
| Stented bioprosthesis | 36(78) | 21(75) | 13(81) | 0.49 |
| Stentless bioprosthesis | 2(4) | 2(7) | 0 | |
| Mechanical valve | 3(7) | 1(4) | 2(13) | |
| Other | 5(11) | 4(14) | 1(6) | |
| Implant device size, mm | 25(23–27) | 25(23–27) | 23(23–25) | 0.11 |
| Concomitant procedures | 30(65) | 14(50) | 14(88) | 0.021 |
| Annular enlargement | 5(11) | 1(4) | 4(25) | 0.06 |
| Mitral | 10(22) | 3(11) | 6(38) | 0.06 |
| Coronary artery bypass grafting | 7(16) | 2(7) | 3(19) | 0.34 |
| Tricuspid | 6(13) | 4(15) | 2(13) | 1.00 |
| Aortic procedure | 15(33) | 7(26) | 8(50) | 0.19 |
| Root repair/replacement | 7(15) | 2(7) | 5(31) | 0.08 |
| Ascending repair/endarterectomy | 12(26) | 7(25) | 5(31) | 0.73 |
| Arch | 3(7) | 3(11) | 0 | 0.29 |
| Ventricular septal defect repair | 1(2) | 1(4) | 0 | 1.00 |
| Multiple concomitant procedures | 8(17) | 2(7) | 5(31) | 0.08 |
| Intra-aortic balloon pump | 3(7) | 2(7) | 1(6) | 1.00 |
| ECMO | 4(9) | 3(11) | 1(6) | 1.00 |
n=44 patients
ECMO, extracorporeal membrane oxygenation
Sixty-five percent of patients (n=30) underwent concomitant procedures during TAVR-explant, including 33% (15/46) undergoing aortic repair/replacement, 22% (10/46) mitral repair/replacement, and 16% (7/46) CABG. Among the 15 patients who underwent a concomitant aortic procedure (n=11 explants of self-expanding devices and n=4 balloon-expandable), 12 underwent ascending repair/replacement (n=9 self-expanding and n=3 balloon-expandable), 7 aortic root repair/replacement (n=6 self-expanding and n=1 balloon-expandable), and 3 aortic arch procedures. A higher proportion of late versus early explants underwent concomitant procedures (88% vs. 50%, p=0.021) [Table 2].
Among patients undergoing non-aortic concomitant procedures with TVT index TAVR data available, 55% (5/9) who underwent concomitant mitral surgery had moderate or worse MR at the time of TAVR, 60% (3/5) undergoing tricuspid repair had moderate or worse TR, and 60% (3/5) undergoing CABG had significant coronary disease.
Post-Explant Outcomes
Mortality in the hospital or within 30 days was 20% (9/46), including 45% (5/11) among patients emergently/urgently converted on the same day as index TAVR. Median postoperative length of stay among those discharged alive was 11 (9–17) days. In total 76% (35/46) of patients had at least one postoperative in-hospital complication, including 37% (17/46) with new postoperative atrial fibrillation, 23% (9/40) new renal failure, 11% (5/46) reoperation for bleeding, 6% (2/33) new permanent pacemaker placement, and 4% (2/46) permanent stroke. Among those alive at discharge, 37% (17/37) were discharged to home and 30-day readmission was 27% (10/37). Postoperative outcomes did not statistically differ between early versus late TAVR-explants (Table 3). TAVR-explants who had undergone prior VIV procedures for failed bioprosthetics had a 0% (0/14) operative mortality, 64% (9/14) had at least one in-hospital complication, 71% (10/14) were discharged home, and 30-day readmission was 14% (2/14).
Table 3.
Postoperative outcomes by timing of TAVR-explant. Values are expressed as number (%) or median (interquartile range).
| Characteristic | Overall (n=46) | Early (<1y, n=28) | Late (>1y, n=16) | P-value |
|---|---|---|---|---|
| Operative mortality | 9(20) | 6(21) | 3(19) | 1.00 |
| ICU length of stay, hours | 113(47–209) | 112(52–172) | 146(45–229) | 0.57 |
| In-hospital complication, % | 35(76) | 20(71) | 13(81) | 0.72 |
| Permanent stroke | 2(4) | 2(7) | 0 | 0.53 |
| Reoperation for bleeding | 5(11) | 2(7) | 3(19) | 0.34 |
| New renal failure† | 9(23) | 4(17) | 4(29) | 0.43 |
| Atrial fibrillation | 17(37) | 11(39) | 5(31) | 0.75 |
| New pacemaker‡ | 2(6) | 0 | 2(17) | 0.14 |
| Postoperative length of stay, days* | 11(9–17) | 10(8–16) | 12(9–25) | 0.23 |
| Discharge location* | ||||
| Home | 17(37) | 9(39) | 8(67) | 0.16 |
| Extended/transitional care/rehab | 19(41) | 14(61) | 4(33) | |
| Nursing home | 1(2) | 0 | 0 | |
| 30-day readmission* | 10(27) | 5(23) | 5(42) | 0.44 |
| All-cause mortality | 15(33) | 9(32) | 6(38) | 0.75 |
| Follow-up after TAVR-explant, months | 1.8(0.7–6.5) | 1.6(0.4–11.0) | 2.0(0.9–5.3) | 0.97 |
n=37 patients alive at discharge
n=40 without end-stage renal disease preoperatively
n=33 without a permanent pacemaker preoperatively
ICU, intensive care unit
All-cause mortality was 33% (15/46) at median 14.1 (2.8–40.8) months follow-up after index TAVR and 1.8 (0.7–6.5) months after TAVR-explant. Estimated survival after TAVR-explant was 73±14% at 3 months, 68±15% at 6 months, and 56±20% at 12 months (eFigure 4).
DISCUSSION
This is the first study using multicenter registry data to comprehensively describe patients undergoing TAVR-explant by linking TVT Registry TAVR procedural and STS ACSD surgical TAVR-explant data. Collectively, these data indicate that TAVR-explants are rare but increasing in frequency, often require concomitant cardiac surgery, and confer significant operative mortality and morbidity.
Prior analyses have described redo TAVR with an incidence of 0.4%1 and 0.33%,2 with a recent international registry analysis reporting an excellent 5.4% 30-day mortality among patients with early (<1 year) valve dysfunction and 1.4% among those with late (>1 year) dysfunction prompting redo TAVR.2 However, these analyses are restricted to transcatheter registry data and do not address the population of TAVR patients with contraindications to redo TAVR. The 0.4% frequency in this study suggests that TAVR-explant may be at least as common as redo TAVR. Tang and colleagues established an interesting model which estimated that redo TAVR after Sapien 3 TAVR would be unfeasible in 21.4% of cases.10 However, the model focuses specifically on the risk of coronary obstruction based on leaflet or stent frame interaction with coronary arteries. The overall feasibility of redo TAVR is likely lower than predicted through this model since the analysis could not consider progression of thrombus, leaflet thickening, and calcification of the native/prosthetic valve or the aortic root over time, potential device constraint of the second TAVR valve, and progression of other synchronous/de novo cardiac pathologies. These factors cannot be appreciated on intraoperative angiogram at the time of index TAVR.6,11 In addition, the requirement for concomitant procedures common in this and prior7 studies presents another contraindication to redo TAVR. Future analyses should evaluate both redo TAVR and TAVR-explant within the same dataset to fully characterize the incidence, indications, and outcomes of transcatheter or surgical reintervention for failed TAVR valves.
Other studies have described TAVR-explants using surgical databases5,7 or Medicare data.8 Interestingly, in an STS ACSD analysis between 2011–2015,5 only 7% of patients underwent root replacement, 2.4% mitral replacement, and 5.7% CABG concomitant to TAVR-explant, compared with 33% who underwent concomitant aortic procedures, 22% mitral, and 16% CABG in this series. These drastic differences may indicate that TAVR-explant procedures became more complicated after 2015, this series may include a higher proportion of late explants, or that regional differences exist between our state and national data. Additionally, 33% of patients in the current study underwent TAV-in-SAV VIV procedures before subsequently requiring TAVR-explant, whereas the STS ACSD database analysis does not include data from the index TAVR and the number of VIV procedures is unknown.5
A more updated STS ACSD analysis including TAVR-explants between 2011–2018 found a 19.4% 30-day mortality among 782 patients, higher among patients undergoing concomitant procedures (23.8%) versus isolated SAVR (14.8%; p=0.002).7 In contrast to the 2011–2015 study with rare concomitant procedures,5 the authors reported ascending aortic or root replacement in 25.6%, mitral surgery in 21.1%, and CABG in 15.6%, which were comparable to the current study. Notably, these analyses were unable to define TAVR-explant frequency due to data limitations, although Jawitz et al.5 estimated a 0.3% TAVR-explant incidence. While one prior study has analyzed data from index TAVR and TAVR-explant within the same dataset and reported a 1.0% TAVR-explant frequency, this was a single-center study with only 17 patients.6 Since the majority of patients in this and prior studies were high-risk when faced with TAVR-explant, we hypothesize that the incidence of TAVR-explant reported here and elsewhere likely underestimates the incidence of failed TAVR valves since some patients were likely not offered redo TAVR or TAVR-explant due to their extremely high-risk status.
Another recent analysis of TAVR-explant procedures in Medicare beneficiaries from 2012–2017 found an incidence of 0.2%.8 The authors reported 8.4% underwent concomitant CABG and 4.4% other valve procedures and a similarly high mortality at 13.2% and 17.6% at 30 and 90 days, respectively.8 However, these data are limited to Medicare beneficiaries and most importantly do not fully capture concomitant procedures at TAVR-explant, such as aortic repair. Given that the rates of concomitant procedures and specifically aortic repair/replacement were 65% and 33% in this study and 55.9% and 25.6% in the nationally-representative STS ACSD analysis,7 capturing these data are important to inform lifetime management of severe aortic stenosis patients and we question whether these patients may have received incomplete therapy at their index TAVR.
Prior multicenter analyses have notably not included TAVR procedural and echocardiographic data, which are essential to understanding why TAVR valves fail and differentiating between concomitant pathology such as MR, TR, or coronary disease being present at the time of TAVR versus developing in the interval between TAVR and TAVR-explant. In this study, 55% (5/9) of patients who underwent concomitant mitral surgery had moderate or worse MR at the initial TAVR. As prior studies have shown, both MS12 and MR13 left untreated at index TAVR are associated with higher mortality. Furthermore, the majority of patients who underwent concomitant tricuspid repair and CABG had moderate or worse TR or significant coronary disease, respectively, at the time of index TAVR. Given the median number of TAVR-explants per surgeon was 1, we expect an associated learning curve (particularly with older TAVR valves), which may contribute to the high reported mortality and morbidity rates. Additionally, the need for other cardiac surgery at time of TAVR-explant was present in more than 25% of patients and may result from incomplete therapy of synchronous cardiac pathology at the initial intervention.
These data raise a concern for the appropriateness of TAVR as a “first valve” strategy in younger, healthier patients who inevitably outlive the lifespan of their TAVR valves. With recent favorable outcomes after TAVR in low-risk patients3.4 and the subsequent Centers for Medicare and Medicaid National Coverage Decision which may double the number of hospitals eligible to perform TAVR,14 the number of TAVR procedures performed in low-risk patients is expected to increase substantially. Although a 0.4% TAVR-explant frequency reinforces similarly low incidences reported with TAVR-explant in Medicare (0.2%)8 and redo TAVR data (0.33%),2 this study importantly does not include any patients undergoing TAVR-explant who were deemed low-risk at their index TAVR.
Limitations
This study has several limitations. First, it is descriptive, with a relatively small sample size. However, this is the only registry analysis providing linked STS and TVT data, which provides unique insights into TAVR-explants which cannot be obtained from STS ACSD or Medicare data alone. Second, insights into operative technique are limited in this database study. However, we include important procedural data from both the TAVR and TAVR-explant procedures and have previously published on TAVR-explant technique in significant detail.6,9 Third, the granularity of TAVR-explant indications is limited to data reported in the STS ACSD, which is a limitation of registry studies. Fourth, follow-up is short for recent TAVR procedures, which comprise the majority of TAVRs performed. As a result, future analyses may show that TAVR-explants occur at later times than represented in these data.
Conclusion
TAVR-explant is a rare, but clinically significant procedure required for some patients with failed TAVR valves. These procedures carry a higher risk of surgical mortality than at the time of index TAVR and two-thirds of patients in this series required concomitant cardiac surgical procedures at the time of TAVR-explant. As the widespread adoption of TAVR continues and the number of younger, lower risk patients become TAVR candidates, providers should consider these data in the context of lifetime management to determine the best initial valve strategy for severe aortic stenosis patients.
Supplementary Material
What is Known:
Repeat TAVR procedures for failed TAVR valves (redo TAVR) have been performed in approximately 0.3–0.4% of patients.
Patients with unsuitable anatomy or other contraindications to redo TAVR may undergo surgical explantation of TAVR bioprotheses in conjunction with surgical aortic valve replacement (TAVR-explant).
What the Study Adds:
By linking data from the Society of Thoracic Surgeons (STS) Adult Cardiac Surgery Database and Transcatheter Valve Therapy (TVT) Registry at Michigan hospitals, this study provides the age of failed TAVR valves and characterizes concurrent coronary and valvular pathology at both the time of index TAVR and TAVR-explant.
The most common indications for TAVR-explant procedures were procedure-related failure, paravalvular leak, and the need for other cardiac surgery.
The most common contraindications to redo TAVR in patients undergoing TAVR-explant were the need for other cardiac surgery, unsuitable non-coronary aortic root anatomy, coronary obstruction, and endocarditis.
ACKNOWLEDGEMENTS
The authors would like to thank every member of the Michigan Society of Thoracic and Cardiovascular Surgeons Quality Collaborative (MSTCVS-QC) and Blue Cross Blue Shield Cardiovascular Consortium (BMC2) Coordinating Centers for their assistance with this study.
SOURCES OF FUNDING
Dr. Brescia is supported by the National Research Service Award postdoctoral fellowship (No. 5T32HL076123).
DISCLOSURES
Support for the Michigan Society of Thoracic and Cardiovascular Surgeons (MSTCVS) Quality Collaborative is provided by the Blue Cross and Blue Shield of Michigan (BCBSM) and Blue Care Network as part of the BCBSM Value Partnerships program. Although BCBSM works collaboratively with MSTCVS-QC, opinions, beliefs, and viewpoints expressed by the authors do not necessarily reflect the opinions, beliefs and viewpoints of BCBSM or any of its employees.
Dr. Fukuhara serves as a consultant for Terumo Aortic. Dr. Deeb was supported by Medtronic Inc. as site Principle Investigator for the Pivotal, Extreme, High, SURTAVI, and Low Risk TAVR trials. Money went to University of Michigan; no personal remuneration was received. Dr. Sukul reported that he is a member of the Society for Cardiovascular Angiography and Interventions government relations committee. All other authors have nothing to disclose with regard to commercial support.
Non-standard Abbreviations and Acronyms
- BCBSM
Blue Cross Blue Shield of Michigan
- BMC2
Blue Cross Blue Shield Cardiovascular Consortium
- ECMO
extracorporeal membrane oxygenation
- MSTCVS-QC
Michigan Society of Thoracic and Cardiovascular Surgeons Quality Collaborative
- STS-PROM
Society of Thoracic Surgeons predicted risk of mortality
- PVL
paravalvular leak
- STS ACSD
Society of Thoracic Surgeons Adult Cardiac Surgery Database
- TVT
Transcatheter Valve Therapy
- VIV
valve-in-valve
REFERENCES
- 1.Barbanti M, Webb JG, Tamburino C, Van Mieghem NM, Makkar RR, Piazza N, Latib A, Sinning J-M, Won-Keun K, Bleiziffer S, et al. Outcomes of Redo Transcatheter Aortic Valve Replacement for the Treatment of Postprocedural and Late Occurrence of Paravalvular Regurgitation and Transcatheter Valve Failure. Circ Cardiovasc Interv 2016;9:e003930. [DOI] [PubMed] [Google Scholar]
- 2.Landes U, Webb JG, De Backer O, Sondergaard L, Abdel-Wahab M, Crusius L, Kim W-K, Hamm C, Buzzatti N, Montorfano M, et al. Repeat Transcatheter Aortic Valve Replacement for Transcatheter Prosthesis Dysfunction. J Am Coll Cardiol 2020;75:1882–1893. [DOI] [PubMed] [Google Scholar]
- 3.Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O’Hair D, Bajwa T, Heiser JC, Merhi W, Kleiman NS, et al. Evolut Low Risk Trial Investigators. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N Engl J Med 2019;380:1706–1715. [DOI] [PubMed] [Google Scholar]
- 4.Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, et al. PARTNER 3 Investigators. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med 2019;380:1695–1705. [DOI] [PubMed] [Google Scholar]
- 5.Jawitz OK, Gulack BC, Grau-Sepulveda MV, Matsouaka RA, Mack MJ, Holmes DR Jr, Carroll JD, Thourani VH, Brennan JM. Reoperation After Transcatheter Aortic Valve Replacement: An Analysis of the Society of Thoracic Surgeons Database. JACC Cardiovasc Interv 2020;13:1515–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukuhara S, Brescia AA, Shiomi S, Rosati CM, Yang B, Kim KM, Deeb GM. Surgical explantation of transcatheter aortic bioprostheses: Results and clinical implications. J Thorac Cardiovasc Surg 2020;S0022–5223(20)30061–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukuhara S, Brescia AA, Deeb GM. Surgical Explantation of Transcatheter Aortic Bioprosthesis: An Analysis from the Society of Thoracic Surgeons Database. Circulation. 2020;142:2285–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirji SA, Percy ED, McGurk S, Malarczyk A, Harloff MT, Yazdchi F, Sabe AA, Bapat VN, Tang GHL, Bhatt DL, et al. Incidence, Characteristics, Predictors, and Outcomes of Surgical Explantation After Transcatheter Aortic Valve Replacement. J Am Coll Cardiol 2020;76:1848–59. [DOI] [PubMed] [Google Scholar]
- 9.Fukuhara S. Safe Late Explantation of Transcatheter Aortic Bioprosthesis. Ann Thorac Surg 2020;110:e555–e558. [DOI] [PubMed] [Google Scholar]
- 10.Tang GHL, Zaid S, Gupta E, Ahmad H, Khan A, Kovacic JC, Lansman SL, Dangas GD, Sharma SK, Kini A. Feasibility of Repeat TAVR After SAPIEN 3 TAVR: A Novel Classification Scheme and Pilot Angiographic Study. JACC Cardiovasc Interv 2019;12:1290–1292. [DOI] [PubMed] [Google Scholar]
- 11.Mangi AA, Ramchandani M, Reardon M. Surgical Removal and Replacement of Chronically Implanted Transcatheter Aortic Prostheses: How I Teach It. Ann Thorac Surg 2018;105:12–14. [DOI] [PubMed] [Google Scholar]
- 12.Kato N, Padang R, Pislaru C, Miranda WR, Hoshino M, Shibayama K, Watanabe H, Scott CG, Greason KL, Pislaru SV, et al. Hemodynamics and Prognostic Impact of Concomitant Mitral Stenosis in Patients Undergoing Surgical or Transcatheter Aortic Valve Replacement for Aortic Stenosis. Circulation. 2019;140:1251–1260. [DOI] [PubMed] [Google Scholar]
- 13.Nombela-Franco L, Ribeiro HB, Urena M, Allende R, Amat-Santos I, DeLarochielliere R, Dumont E, Doyle D, DeLarochielliere H, Laflamme J, et al. Significant mitral regurgitation left untreated at the time of aortic valve replacement: a comprehensive review of a frequent entity in the transcatheter aortic valve replacement era. J Am Coll Cardiol 2014;63:2643–2658. [DOI] [PubMed] [Google Scholar]
- 14.Thompson MP, Brescia AA, Hou H, Pagani FD, Sukul D, Dimick JB, Likosky DS. Access to Transcatheter Aortic Valve Replacement Under New Medicare Surgical Volume Requirements. JAMA Cardiol 2020;5:729–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.