Abstract
Background:
Decreased cholinergic tone associated with increased proinflammatory cytokines has been observed in several human diseases associated with low-grade inflammation. We examined if this attenuated cholinergic anti-inflammatory pathway (CAP) mechanism contributes to increased neuroinflammation observed in depression.
Methods:
We measured cerebrospinal fluid (CSF) cholinergic markers (AChE and BChE activities) in 28 individuals with longstanding late-life major depression (LLMD) and 19 controls and their relationship to central and peripheral levels of pro-inflammatory cytokines (IL-6 and IL-8). Additionally, we examined if these cholinergic indices were related to CSF markers of microglial activation and neuroinflammation (sTREM2 and complement C3).
Results:
Compared with controls, LLMD patients had a significant reduction in CSF BChE levels. Lower CSF BChE and AChE activities were associated with lower CSF markers of microglial and neuroinflammation (sTREM2 and C3). In addition, in LLMD patients we found an inverse relationship between peripheral marker of inflammation (plasma IL-6) and CSF BChE and AChE levels.
Conclusions:
Our results suggest an upregulation of the CAP mechanism in LLMD with an elevation in peripheral markers of inflammation and concomitant reduction in markers of glial activation associated with a higher cholinergic tone. Future studies should confirm these findings in a larger sample including individuals with acute and more severe depressive episodes and across all ages.
Keywords: Cholinergic anti-inflammatory pathway, cerebrospinal fluid, acetylcholinesterase, butyrylcholinesterase, C3, late-life major depression
Introduction
An increased inflammatory state has been implicated in the pathophysiology of depression and numerous reports and meta-analyses have documented increases of various peripheral proinflammatory cytokines, such as interleukin 6 (IL-6) and interleukin 8 (IL-8) in depression, including late-life major depression (LLMD) (Bremmer et al., 2008; Felger and Lotrich, 2013; Köhler et al., 2017; Maes, 1995, 2008; Martínez-Cengotitabengoa et al., 2016; Raison and Miller, 2011; Syed et al., 2018; Young et al., 2014). These abnormalities are particularly prominent in drug-naïve individuals during their first episode of depression, in those with severe depression, suicidal ideation or a history of child abuse, and in patients with treatment-resistant depression (Maes, 2008; Raison and Miller, 2011). It has been hypothesized that increases in peripheral inflammation may be etiologically linked to depression (Felger and Lotrich, 2013; Maes, 2008; Raison and Miller, 2011).
Increases in peripheral cytokine levels may elicit activation of a cholinergic anti-inflammatory response (Rosas-Ballina et al., 2011; Tracey, 2002). Enhancement o f central cholinergic tone with cholinesterase inhibitors may reduce the release of pro-inflammatory cytokines (Pavlov et al., 2009). This anti-inflammatory effect is mediated by the stimulation of α7 nicotinic acetylcholine receptors (α7 nAChR) on macrophages which suppresses the activation of the nuclear factor kappa B (NF-kB) signal transcription pathway (Borovikova et al., 2000; Rosas-Ballina et al., 2011; Wang et al., 2003). In low grade inflammatory disorders, such as type 2 diabetes and hypertension, increased acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) activity is associated with reduced acetylcholine (ACh) concentrations and increased pro-inflammatory cytokines (Das, 2007). Since depression is also associated to low grade inflammation, we evaluated the cholinergic anti-inflammatory pathway (CAP) in a group of subjects with late-life major depression (LLMD). Specifically, we examined whether cerebrospinal fluid (CSF) AChE and BChE activities were altered in LLMD, and whether they were related to central and peripheral levels of pro-inflammatory cytokines (IL-6 and IL-8). In addition, we examined whether CSF AChE and BChE were linked to CSF soluble triggering receptor expressed on myeloid cells 2 (sTREM2), a marker of activated microglia (Suárez- Calvet et al., 2016), and CSF complement component 3 (C3), a marker of inflammation as these indices may be especially sensitive to a loss of brain cholinergic signaling and downregulation of CAP (Schmitz et al., 2020).
Methods
Setting
This study was a longitudinal evaluation of elderly depressed in the community, who were followed for a period of 3 years. The study was conducted at two research sites: at a Geriatric Psychiatry Clinic at the Nathan S. Kline Institute (NKI) and at the NYU Langone Medical Center. For more information on the setting, please see Supplemental Methods.
Participants
This study was approved by the institutional review boards of the NKI and New York University School of Medicine (NYU SoM). Participants were community volunteers that were recruited through advertisements and the MERI program at NKI. A total of 192 participants were recruited and 59 participants were excluded after the screening visit. Participants were excluded if they did not meet any of the following inclusion criteria: a) Cognitively intact individuals, b) 60 years or older, c) A score of < 25 on the Mini-Mental State Examination (MMSE), d) A CDR global score of 0, or e) clinically abnormal lab values.
A total of 133 participants completed baseline evaluation and 51 completed the optional lumbar puncture (LP). The analysis presented in this paper focuses on the LP group. Of the 51 participants who performed the LP, three were excluded due to evidence of confluent deep or periventricular white matter hyperintensities (one or more hyper-intense lesions measuring at least 10 mm in any direction) from magnetic resonance imaging (MRI). One participant was excluded because of a MMSE score below 28 to control for possible early cognitive impairment. Of the remaining 47 participants, 28 were diagnosed with Major Depressive Disorder (MDD) by a board-certified psychiatrist using the Structural Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV disorders (SCID) and 19 served as aged-match controls. A detailed description of this sample can be found in Pomara et al. (2012). Finally, CSF AChE and BChE levels were only determined in 27 depressed and 17 control participants. Of the 27 remaining individuals with MDD, 20 (74%) had recurrent episodes. Please see Table 1 for a summary of the demographic and clinical characteristics of the study participants.
Table 1. Demographics and Biological Variables for the Study group.
The study group includes LLMD (N = 27) and Controls (N = 17). The percent of the total sample are reported for sex and APOE status. The mean, SD and group-comparison are reported for age, education, Hamilton Depression Rating Scale (HAM-D), and Mini-Mental State Examination score are listed under demographics. Significant differences were found only on the HAM-D (p < 0.001), there were no other significant group differences for the demographic or clinical variables. The means and SDs for the biological variables are listed. As reported in the Results section, there was a significant difference between the LLMD and controls for BChE.
| Control Mean (SD) | LLMD Mean (SD) | ||
| N | 17 | 27 | |
| Sex (female, % of total) | 10 (58.8) | 9 (33.3) | |
| APOE Status (positive, % of total) | 3 (17.6) | 11 (40.7) | |
| Demographics | Independent Samples T-Test (p-value) | ||
| Age (years) | 67.65 (6.74) | 66.67 (5.36) | p = 0.60 |
| Education (years) | 16.59 (2.79) | 16.44 (2.70) | p = 0.87 |
| MMSE (score) - Screening Visit | 29.47 (0.51) | 29.74 (0.66) | p = 0.16 |
| Hamilton Depression Rating Scale (Score) - Screening Visit | 1.12 (1.90) | 16.56 (10.22) | p < 0.001* |
| Biological Variables | |||
| CSF AChE (mU/mL) | 60.85 (15.79) | 53.13 (19.07) | |
| CSF BChE (mU/mL) | 37.50 (10.35) | 29.34 (11.70) | |
| CSF IL-6 (pg/mL) | 4.87 (5.27) | 4.53 (3.08) | |
| CSF IL-8 (pg/mL) | 96.46 (32.75) | 86.62 (15.40) | |
| CSF C3 (ng/mL) | 12247.78 (8377.14) | 8187.21 (4805.54) | |
| CSF sTREM2 (pg/mL) | 5361.55 (2802.26) | 3609.23 (2691.04) | |
| Plasma IL-6 (pg/mL) | 2.46 (1.50) | 2.20 (1.46) | |
| Plasma IL-8 (pg/mL) | 5.24 (2.73) | 7.33 (3.96) | |
indicates signifcant difference
Procedure
The first three visits were conducted at NKI and/or the NYU SoM. During the first visit, participants were given informed consent forms along with information on the study procedures and their rights. The participants’ medical and psychiatric histories were collected, and vital signs were measured. A psychiatric evaluation was then carried out while the MMSE was administered for global cognitive status. The Hamilton Depression Rating Scale (HAM-D) was administered to rate the severity of current depressive symptoms. Participants who met the criteria for past MDD but were not currently depressed (with a score less than 16 on the HAM-D) were included as MDD subjects. Lastly, blood was drawn for routine medical testing.
During the second visit, participants completed a MRI scan of the head. During the third visit, participants underwent a comprehensive neuropsychological assessment. Lastly, during the fourth visit, an optional LP was done in participants who consented. For more information on the study procedures, please see Supplemental Methods.
Protein and Cytokine Determination
The lumbar puncture required participants to fast overnight and was performed between 0900 and 1000 hours. A total of 15 mL of clear CSF was collected in three polypropylene tubes. The tubes were immediately placed on ice for a maximum of 1 hour until samples were centrifuged at 4°C at 1500 rpm for 10 min. Aliquots of 0.25 mL were then placed into 1 mL polypropylene cryogenic vials and stored in Nunc eight-cell storage boxes (Nalge Nunc Internation, Rochester, NY, USA) at -80°C.
CSF and plasma levels of IL-6 and IL-8 were measured in a sandwich assay format using the Human Proinflammatory II 4-Plex Assay and a SECTOR instrument from Meso Scale Discovery Gaithersburg, MD. CSF AChE and BChE activities were determined using an inhouse method as previously described (Parnetti et al., 2011). CSF Abeta and tau determinations were described previously (Pomara et al., 2012). sTREM2 concentration was determined using an electrochemiluminescence immunoassay as previously described (Heslegrave et al., 2016). CSF C3 levels were measured using a Complement C3 Human ELISA kit (cat #:ab108823; abcam) as previously described (Pillai et al., 2019) and values are expressed as ng/ml. The focus of this analysis will be on the baseline lumbar puncture determination.
Statistical Analysis
Wilcoxon Rank sum tests were carried out to examine differences in CSF AChE and BChE between LLMD and controls. Spearman’s correlations were then applied to examine associations between CSF AChE and BChE, and CSF (Il6, IL8, Aβ40, Aβ42, total-tau, phospho-tau, C3 and sTREM2) and plasma (IL-6 and IL-8) biomarkers. Kruskal-Wallis test was used to examine the effect of antidepressant status on AChE and BChE. Statistical analysis was conducted in SPSS version 24.0. Regression (Spearman) plots were generated using Seaborn v0.10.0. The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Results
Demographic characteristics and the biological variables of interest for the study group are shown in Table 1. The original study group consisted of 27 LLMD and 17 cognitively normal controls. Significant differences between the two groups were found only on the HAM-D score (p < 0.001). There were no other significant group differences for the demographic variables.
CSF AChE levels were marginally-significantly lower in LLMD compared to controls for (Figure 1a; p = 0.061). As shown in Figure 1b, CSF BChE levels were significantly lower in individuals with LLMD compared to controls (p = 0.008). In LLMD, there was also a significant positive correlation between CSF AChE levels and BChE levels (rho = 0.598, p < 0.001).
Figure 1. Group differences for CSF cholinesterase activity.
Boxplots comparing the study groups Controls (orange) vs. LLMD ( blue) withdata points superimposed. a) CSF AChE levels were marginally significantly lower than controls (p = 0.061) b) CSF BChE levels were significantly lower in LLMD (p = 0.008). Wilcoxon Rank sum test for the group comparisons of biomarker levels. P values are shown.
In LLMD, lower CSF BChE and AChE were associated with higher plasma IL-6 (Figure 2a and 2b; AChE: rho = -0.459, p = 0.016; BChE: rho = -0.449, p = 0.019). In contrast, in the control group, only lower CSF AChE was associated with higher plasma IL-8 (Figure 2c; rho = 0.542, p = 0.025).
Figure 2. Correlations between CSF cholinesterase AChE and BChE with Plasma IL-6 and IL-8 for LLMD and Controls.
Regression plots (Spearman) comparing LLMD with controls for AChE (a) and BChE (b) with Plasma IL-6 levels. Lower CSF BChE (r = -0.449, p = 0.019) and AChE (r = -0.459, p = 0.016) were associated with higher plasma IL-6 for LLMD only. Regression plots (Spearman) comparing LLMD with controls for AChE (c) and BChE (d) with Plasma IL-8. For the control group only, lower CSF AChE was associated with higher plasma IL-8 (r = -0.542, p = 0.025).
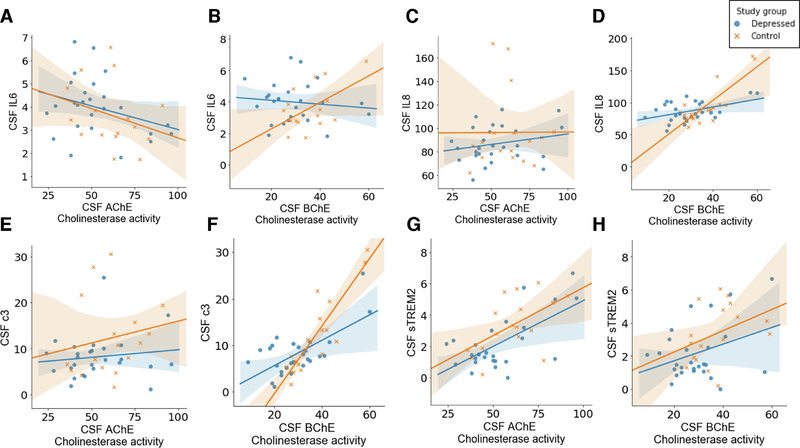
In both depressed individuals and controls, CSF BChE was directly associated with CSF C3 (Figure 3f; LLMD: rho = 0.423, p = 0.028, Controls: rho = 0.813, p < 0.001). CSF BChE, in controls, was also positively associated with both CSF IL8 and CSF sTREM2 (Figure 3d and 3h respectively; IL-8: rho = 0.621, p = 0.008, sTREM2: rho = 0.502, p = 0.040). CSF AChE was associated with CSF sTREM2 in both individuals with LLMD and controls (Figure 3g; LLMD: rho = 0.383, p = 0.049, Controls: rho 0.494, p = 0.044).
Figure 3. Correlations between CSF cholinesterase AChE and BChE with CSF biomarkers (IL6, IL-8, C3, and sTREM2) for LLMD and Controls.
The CSF measurement was taken for a smaller subset of participants, LLMD (N=28) and Controls (N=19). Regression (Spearman) plots comparing LLMD with controls for AChE with CSF IL-6 (a) with CSF IL-8 (c), with CSF C3 (e), and with sTREM-2 (g). For LLMD (r = 0.383, p = 0.049) and Controls (r = 0.494, p = 0.044), CSF AChE was associated with higher sTREM2. Regression (Spearman) plots comparing LLMD with controls for BChE with CSF IL-6 (b), with CSF IL-8 (d), with CSF C3 (f), and with TREM-2 (h). CSF BChE was correlated with C3 in both the LLMD group (r = 0.423, p = 0.028) and Controls (r = 0.813, p < 0.001).
An examination of the effect of antidepressant status (on medication vs. not on medication) on CSF AChE or BChE activity, failed to reveal a significant effect of antidepressant use.
Discussion
In this study, we found that the LLMD group had a significant reduction in BChE activity in CSF compared with controls. However, we found no significant differences in CSF AChE levels across groups as previously reported (Deutsch et al., 1983). There was no significant effect of antidepressant status on CSF BChE nor AChE activity. In the LLMD cohort, lower CSF AChE and BChE levels were associated with higher plasma IL-6, one of the pro-inflammatory cytokines frequently reported to be elevated in depression (Enache et al., 2019). We also found significant positive correlations between CSF BChE or AChE and CSF cytokines, C3 and sTREM2.
Elevations in tissue and circulating AChE and BChE and increased proinflammatory cytokines are consistent with an attenuation of the CAP and have been reported in a number of diseases, which like depression, are associated with low grade inflammation and elevation in proinflammatory cytokines (Das, 2007). In our study we did not observe any increase in CSF AChE nor BChE activities in LLMD compared to controls, rather CSF BChE was significantly reduced in LLMD. Surprisingly, lower CSF BChE and AChE activities were associated with higher plasma IL-6 levels, consistent with an upregulation rather than an attenuation of CAP in LLMD in response to systemic inflammation.
Neither CSF AChE nor BChE has been associated with indices of brain cholinergic activity. In rats, destruction of basal cholinergic neurons did not cause significant changes in CSF levels of these enzymes (Roiner et al., 1998). In patients with Alzheimer’s disease (Appleyard and McDonald, 1992; Bisso et al., 1986; Marquis et al., 1985) and in patients with other neurodegenerative disorders associated with extensive loss of brain cholinergic neurons (Ruberg et al., 1987), both CSF AChE and BChE activity appear normal. Since BChE is expressed in a very small number of cholinergic neurons (Mesulam et al., 2002), the reduction in CSF BChE that we observed in LLMD is unlikely to translate in higher synaptic acetylcholine levels and upregulation of cholinergic neuronal activity. Consistent with other CSF studies (Aeinehband et al., 2015; Darreh-Shori et al., 2013), CSF AChE and BChE were positively correlated with CSF sTREM2 and C3, respectively. Since both AChE and BChE are expressed in glial cells (Mesulam et al., 2002), their CSF levels are more likely to reflect glial cell activation rather than cholinergic neuronal activity. The reduction in CSF BChE that we observed in LLMD, the positive correlations between CSF AChE and BChE with microglial (sTREM2) and inflammatory (C3) markers and their negative correlations with plasma cytokines, might reflect an upregulation of CAP in response to an increase in systemic inflammation.
To our knowledge, there are no studies which have examined the relationship between potential indices of CAP activation, such as AChE and BChE activity, and inflammatory markers in depression. Two studies examined the relationship between the inflammatory cytokine IL-1β and expression of α7nAChR in peripheral mononuclear blood cells in individuals with eating and personality disorders (Díaz-Marsá et al., 2012; MacDowell et al., 2013). These studies found elevations in the inflammatory indices associated with increased expression of α7nAChR receptors. Acetylcholine-mediated stimulation of these receptors on the surface of inflammatory cells is associated with inhibition of NF-kβ nuclear translocation and suppression of pro-inflammatory cytokine suppression. These findings were interpreted as reflecting an upregulation of CAP; hence, they complement our results and taken together they suggest that both the central and peripheral arms of CAP may be activated in humans in response to systemic inflammation.
In preclinical and in vitro experiments, selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants (TCAs) have been shown to inhibit human BChE and AChE activity (Müller et al., 2002). Our analyses did not reveal a clear effect of antidepressant use on CSF BChE nor AChE activity. However, given the relatively small sample size, the possibility that antidepressant treatment might have contributed to the reduction in CSF BChE and upregulation of the cholinergic anti-inflammatory response in LLMD cannot be completely excluded.
The existing literature suggests that elevations in pro-inflammatory markers are not an invariant finding in depression (Raison and Miller, 2011). Only about one third of depressed individuals have been reported to have plasma cytokines higher than heathy controls. It is also not known how many of the depressed individuals with elevated plasma cytokine levels will have evidence of increased neuroinflammation. Studies using in vivo markers of neuroinflammation such as various translocator protein (TSPO)-PET ligands, have provided conflicting data. Some studies, including a study in geriatric depression, have found evidence consistent with increased neuroinflammation, while others have reported no change and one found numerical reductions in diverse brain regions compared to controls (Hannestad et al., 2013; Su et al., 2016). Indeed, both increa sed and decreased microglial activation which might reflect variability in CAP upregulation have been implicated in the pathogenesis depression and different inflammation-based treatment approaches have been proposed for these distinct depression subtypes (Yirmiya et al., 2015).
Our study has limitations. One is that the sample size is relatively small, with slightly uneven distribution of LLMD and controls. Additionally, the level of severity of depression was different among the LLMD individuals. It is possible that the differences reported above may be further modulated by the degree of severity; therefore, future studies should try to match degree of severity. All depressed patients were 60 years and older and suffered from long-standing depression. None showed levels of pro-inflammatory cytokine that would be considered clinically significant. Thus, it is not known if our results are pertinent to younger populations and those with acute depression and significant systemic inflammation. The use and type of medication were also not controlled for in the LLMD group. Although the effect of medication was explored, these medications could have differentially affected the biological markers. Future studies should consider controlling for medication type.
In conclusion, our preliminary results suggest that upregulation, rather than downregulation of the cholinergic anti-inflammatory pathway, may be associated with increased systemic inflammation in LLMD. Interestingly acute administration of the antimuscarinic agent scopolamine has been found to be associated with rapid antidepressant response in treatment-resistant patients, and there is a large body of observations implicating excessive central cholinergic tone in the pathophysiology of depression (Furey and Drevets, 2006). Similarly reduced astrocytic glutamate uptake from increased cholinergic signaling might also result in excessive extracellular glutamate levels which have also been implicated in treatment-resistant depression responsive to the NMDA receptor antagonist ketamine. Thus, future studies should confirm this finding and determine its possible role in the pathophysiology of depression and treatment response.
Supplementary Material
Highlights.
Lower CSF AChE and/or BChE activities may be related to increased cholinergic tone.
Patients with late-life depression show lower CSF BChE activity compared to controls.
Lower CSF AChE and BChE activities are related to higher peripheral inflammation.
They are also related to lower markers of glial activation (sTREM2 and C3).
Acknowledgements
We would like to thank Drs. Antero Sarreal, Raymundo Hernando and Jay Nierenberg for assisting in various aspects of the study.
Funding
This work was supported in part by National Institute of Mental Health (NIMH) grant [R01 MH-080405] to Nunzio Pomara.
Role of Funding Source
Henrik Zetterberg is a Wallenberg Academy Fellow supported by grants from the Swedish Research Council (#2018–02532), the European Research Council (#681712) and Swedish State Support for Clinical Research (#ALFGBG-720931) and the UK Dementia Research Institute at UCL. Anilkumar Pillai’s research is supported by grants from National Institute of Health/National Institute of Mental Health (MH120876 and MH121959) and the Merit Review Award (BX004758) from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development. Kaj Blennow is supported by the Swedish Research Council (#2017–00915), the Alzheimer Drug Discovery Foundation (ADDF), USA (#RDAPB-201809–2016615), the Swedish Alzheimer Foundation (#AF-742881), Hjärnfonden, Sweden (#FO2017–0243), the Swedish state under the agreement between the Swedish government and the County Councils, the ALF-agreement (#ALFGBG-715986).
Financial Disclosure
Henrik Zetterberg is a Wallenberg Academy Fellow supported by grants from the Swedish Research Council (#2018–02532), the European Research Council (#681712) and Swedish State Support for Clinical Research (#ALFGBG-720931) and the UK Dementia Research Institute at UCL. Kaj Blennow is supported by the Swedish Research Council (#2017–00915), the Alzheimer Drug Discovery Foundation (ADDF), USA (#RDAPB-201809–2016615), the Swedish Alzheimer Foundation (#AF-742881), Hjärnfonden, Sweden (#FO2017–0243), the Swedish state under the agreement between the Swedish government and the County Councils, the ALF-agreement (#ALFGBG-715986).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
HZ has served at scientific advisory boards for Roche Diagnostics, Samumed, Wave and CogRx, has given lectures in symposia sponsored by Alzecure and Biogen, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB, a GU Ventures-based platform company at the University of Gothenburg. KB has served as a consultant or at advisory boards for Abcam, Axon, Biogen, Lilly, MagQu, Novartis and Roche Diagnostics, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB, a GU Venture-based platform company at the University of Gothenburg, all unrelated to the work presented in this paper.
Data Availability
The data generated during the current study are not publicly available because the approved IRB protocol does not include a provision for deposit in a public repository.
References
- Aeinehband S, Lindblom RP, Al Nimer F, Vijayaraghavan S, Sandholm K, Khademi M, Olsson T, Nilsson B, Ekdahl KN, Darreh-Shori T, 2015. Complement component C3 and butyrylcholinesterase activity are associated with neurodegeneration and clinical disability in multiple sclerosis. PLoS One 10, e0122048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleyard ME, McDonald B, 1992. Acetylcholinesterase and butyrylcholinesterase activities in cerebrospinal fluid from different levels of the neuraxis of patients with dementia of the Alzheimer type. Journal of Neurology, Neurosurgery & Psychiatry 55, 1074–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisso G, Masullo C, Michalek H, Silveri MC, Pocchiari M, 1986. Molecular forms of cholinesterases in CSF of Alzheimer’s disease/senile dementia of Alzheimer type patients and matched neurological controls. Life sciences 38, 561–567. [DOI] [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ, 2000. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405, 458. [DOI] [PubMed] [Google Scholar]
- Bremmer M, Beekman A, Deeg D, Penninx B, Dik M, Hack C, Hoogendijk W, 2008. Inflammatory markers in late-life depression: results from a population-based study. Journal of affective disorders 106, 249–255. [DOI] [PubMed] [Google Scholar]
- Darreh-Shori T, Vijayaraghavan S, Aeinehband S, Piehl F, Lindblom RP, Nilsson B, Ekdahl KN, Långström B, Almkvist O, Nordberg A, 2013. Functional variability in butyrylcholinesterase activity regulates intrathecal cytokine and astroglial biomarker profiles in patients with Alzheimer’s disease. Neurobiology of aging 34, 2465–2481. [DOI] [PubMed] [Google Scholar]
- Das UN, 2007. Acetylcholinesterase and butyrylcholinesterase as possible markers of low-grade systemic inflammation. Medical Science Monitor 13, RA214–RA221. [PubMed] [Google Scholar]
- Deutsch SI, Mohs R, Levy M, Rothpearl A, Stockton D, Horvath T, Coco A, Davis K, 1983. Acetylcholinesterase activity in CSF in schizophrenia, depression, Alzheimer’s disease, and normals. Biological psychiatry. [PubMed] [Google Scholar]
- Díaz-Marsá M, MacDowell KS, Guemes I, Rubio V, Carrasco JL, Leza JC, 2012. Activation of the cholinergic anti-inflammatory system in peripheral blood mononuclear cells from patients with borderline personality disorder. Journal of psychiatric research 46, 1610–1617. [DOI] [PubMed] [Google Scholar]
- Enache D, Pariante C, Mondelli V, 2019. Markers of central inflammation in major depressive disorder: A systematic review and meta-analysis of studies examining cerebrospinal fluid, positron emission tomography and post-mortem brain tissue. Brain, behavior, and immunity. [DOI] [PubMed] [Google Scholar]
- Felger JC, Lotrich FE, 2013. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience 246, 199–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furey ML, Drevets WC, 2006. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Archives of general psychiatry 63, 1121–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannestad J, DellaGioia N, Gallezot J-D, Lim K, Nabulsi N, Esterlis I, Pittman B, Lee J-Y, O’Connor KC, Pelletier D, 2013. The neuroinflammation marker translocator protein is not elevated in individuals with mild-to-moderate depression: a [11C] PBR28 PET study. Brain, behavior, and immunity 33, 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslegrave A, Heywood W, Paterson R, Magdalinou N, Svensson J, Johansson P, Öhrfelt A, Blennow K, Hardy J, Schott J, 2016. Increased cerebrospinal fluid soluble TREM2 concentration in Alzheimer’s disease. Molecular neurodegeneration 11, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler C, Freitas T, Maes M.d., De Andrade N., Liu C., Fernandes B., Stubbs B., Solmi M., Veronese N., Herrmann N., 2017. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatrica Scandinavica 135, 373–387. [DOI] [PubMed] [Google Scholar]
- MacDowell KS, Díaz-Marsá M, Güemes I, Rodríguez A, Leza JC, Carrasco JL, 2013. Inflammatory activation and cholinergic anti-inflammatory system in eating disorders. Brain, behavior, and immunity 32, 33–39. [DOI] [PubMed] [Google Scholar]
- Maes M, 1995. Evidence for an immune response in major depression: a review and hypothesis. Progress in Neuro-Psychopharmacology and Biological Psychiatry 19, 11–38. [DOI] [PubMed] [Google Scholar]
- Maes M, 2008. The cytokine hypothesis of depression: inflammation, oxidative & nitrosative stress (IO&NS) and leaky gut as new targets for adjunctive treatments in depression. Neuroendocrinol Lett 29, 287–291. [PubMed] [Google Scholar]
- Marquis JK, Volicer L, Mark K, Direnfeld L, Freedman M, 1985. Cholinesterase activity in plasma, erythrocytes, and cerebrospinal fluid of patients with dementia of the Alzheimer type. Biological psychiatry 20, 605–610. [DOI] [PubMed] [Google Scholar]
- Martínez-Cengotitabengoa M, Carrascón L, O’Brien JT, Díaz-Gutiérrez M-J, Bermúdez-Ampudia C, Sanada K, Arrasate M, González-Pinto A, 2016. Peripheral inflammatory parameters in late-life depression: a systematic review. International journal of molecular sciences 17, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M, Guillozet A, Shaw P, Quinn B, 2002. Widely spread butyrylcholinesterase can hydrolyze acetylcholine in the normal and Alzheimer brain. Neurobiology of disease 9, 88–93. [DOI] [PubMed] [Google Scholar]
- Müller TC, Rocha JBT, Morsch VM, Neis RT, Schetinger MR, 2002. Antidepressants inhibit human acetylcholinesterase and butyrylcholinesterase activity. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease 1587, 92–98. [DOI] [PubMed] [Google Scholar]
- Parnetti L, Chiasserini D, Andreasson U, Ohlson M, Hüls C, Zetterberg H, Minthon L, Wallin Å, Andreasen N, Talesa V, 2011. Changes in CSF acetyl-and butyrylcholinesterase activity after long-term treatment with AChE inhibitors in Alzheimer’s disease. Acta Neurologica Scandinavica 124, 122–129. [DOI] [PubMed] [Google Scholar]
- Pavlov VA, Parrish WR, Rosas-Ballina M, Ochani M, Puerta M, Ochani K, Chavan S, Al-Abed Y, Tracey KJ, 2009. Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain, behavior, and immunity 23, 41–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai A, Bruno D, Nierenberg J, Pandya C, Feng T, Reichert C, Ramos-Cejudo J, Osorio R, Zetterberg H, Blennow K, 2019. Complement component 3 levels in the cerebrospinal fluid of Cognitively Intact Elderly Individuals with Major Depressive Disorder. Biomarkers in Neuropsychiatry 1, 100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomara N, Bruno D, Sarreal AS, Hernando RT, Nierenberg J, Petkova E, Sidtis JJ, Wisniewski TM, Mehta PD, Pratico D, 2012. Lower CSF amyloid beta peptides and higher F2-isoprostanes in cognitively intact elderly individuals with major depressive disorder. American Journal of Psychiatry 169, 523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Miller AH, 2011. Is depression an inflammatory disorder? Current psychiatry reports 13, 467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiner S, Bakinde N, Zeitschel U, Schliebs R, Bigl V, 1998. Cerebrospinal fluidCHOLINESTERASES— MARKERS for loss ofcholinergic basal forebrain neurons? International journal of developmental neuroscience 16, 669–673. [DOI] [PubMed] [Google Scholar]
- Rosas-Ballina M, Olofsson PS, Ochani M, Valdés-Ferrer SI, Levine YA, Reardon C, Tusche MW, Pavlov VA, Andersson U, Chavan S, 2011. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334, 98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruberg M, Villageois A, Bonnet A-M, Pillon B, Rieger F, Agid Y, 1987. Acetylcholinesterase and butyrylcholinesterase activity in the cerebrospinal fluid of patients with neurodegenerative diseases involving cholinergic systems. Journal of Neurology, Neurosurgery & Psychiatry 50, 538–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz TW, Soreq H, Poirier J, Spreng RN, 2020. Longitudinal Basal Forebrain Degeneration Interacts with TREM2/C3 Biomarkers of Inflammation in Presymptomatic Alzheimer’s Disease. Journal of Neuroscience 40, 1931–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L, Faluyi YO, Hong YT, Fryer TD, Mak E, Gabel S, Hayes L, Soteriades S, Williams GB, Arnold R, 2016. Neuroinflammatory and morphological changes in late-life depression: the NIMROD study. The British journal of psychiatry 209, 525–526.27758838 [Google Scholar]
- Suárez-Calvet M, Kleinberger G, Caballero MÁA,Brendel M, Rominger A, Alcolea D, Fortea J, Lleó A, Blesa R, Gispert JD, 2016. sTREM2 cerebrospinal fluid levels are a potential biomarker for microglia activity in early-stage Alzheimer’s disease and associate with neuronal injury markers.EMBO molecular medicine 8, 466–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed SA, Beurel E, Loewenstein DA, Lowell JA, Craighead WE, Dunlop BW, Mayberg HS, Dhabhar F, Dietrich WD, Keane RW, 2018. Defective inflammatory pathways in never-treated depressed patients are associated with poor treatment response. Neuron 99, 914–924. e913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey KJ, 2002. The inflammatory reflex. Nature 420, 853. [DOI] [PubMed] [Google Scholar]
- Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, 2003. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature 421, 384. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Rimmerman N, Reshef R, 2015. Depression as a microglial disease. Trends in neurosciences 38, 637–658. [DOI] [PubMed] [Google Scholar]
- Young JJ, Bruno D, Pomara N, 2014. A review of the relationship between proinflammatory cytokines and major depressive disorder. Journal of affective disorders 169, 15–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated during the current study are not publicly available because the approved IRB protocol does not include a provision for deposit in a public repository.