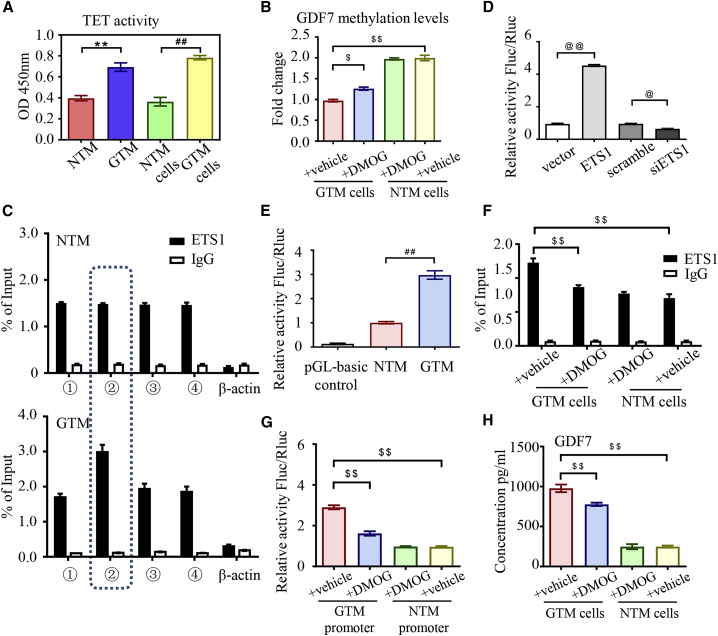
Figure 3.
Ten-eleven translocation (TET) activation promoted glaucomatous GDF7 production
(A) The activity of the TET enzyme was measured in nucleus extracts from TM samples/cells by chemiluminescence assay (n = 3 per group). (B) BSP assay was recruited to measure the methylation level of the GDF7 promoter in response to dimethyloxallyl glycine (DMOG) in NTM and GTM cells (n = 3 per group). (C) All of the RNA fragments that bond to the ETS1 protein were pulled down by ChIP and then tested by qPCR. The amplification of different regions in the GDF7 promoter was conducted with specially designed primers (details in Table S7; n = 3 per group). (D) Luciferase activity was measured to indicate the binding affinity between the GDF7 promoter and ETS1 (n = 3 per group). (E) The binding between ETS1 and the GDF7 promoter from NTM or GTM cells was tested by luciferase reporter assay (n = 3 per group). (F) As measured by ChIP-qPCR, the binding between the ETS1 protein and the GDF7 promoter presented in GTM cells compared with NTM cells in response to DMOG (n = 3 per group). (G) The transcription activity of the GDF7 promoter region in NTM cells and GTM was tested by the luciferase reporter assay. The transcription activity disturbance in response to DMOG was also measured (n = 3 per group). (H) GDF7 secretion was tested in conditioned medium by ELISA. GDF7 secretion was dramatically increased in GTM cells, which was effectively prevented by DMOG treatment (n = 3 per group). The data were represented as mean ± SD. Compared with NTM samples: ∗∗p < 0.01. Compared with NTM cells: ##p < 0.01. Compared with vector or scramble: @p < 0.05, @@p < 0.01. Compared with GTM cells: $p < 0.05, $$p < 0.01. siETS1, siRNA of ETS1.