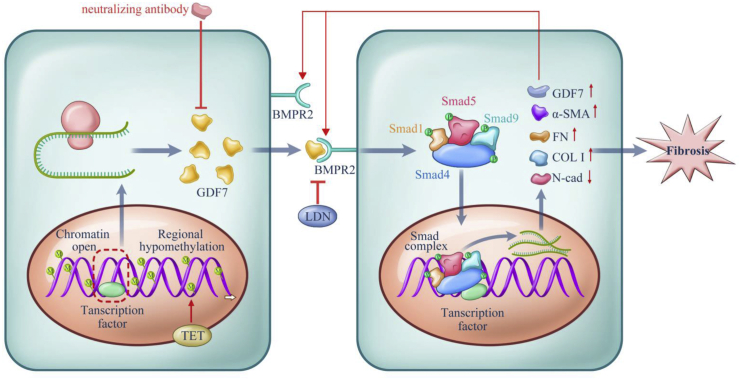
Figure 8.
Proposed model for the methylation-dependent pathology in POAG
Hypomethylation in the GDF7 gene gave rise to the increased GDF7 mRNA transcription via facilitating transcription factors binding to open chromatin. The TET enzyme sustained GDF7 overexpression by keeping the promoter region hypomethylated and open. Excessive GDF7 proteins activated BMPR2 and phosphorylated the downstream effectors, Smad1, -5, and -9 and Smad4. The phosphorylated Smad complex translocated into the nucleus and further promoted the overexpression of GDF7 and fibrosis markers, including Col I, α-SMA, and FN. Meanwhile, the expression of N-cad was suppressed, which remains to be explored in further studies. The imbalance between these proteins triggered TM fibrosis, which leads to the function failure in TM and ended up in IOP elevation. The accumulated GDF7 protein also activated the TET enzyme via an unknown mechanism, thus forming a positive feedback loop. Based on the aforementioned mechanism, the nGDF7 could impede the pro-fibrotic effect of GDF7. Therefore, GDF7 neutralization halts the progression toward fibrosis and maintains a normal AH through the TM.