Figure 3.
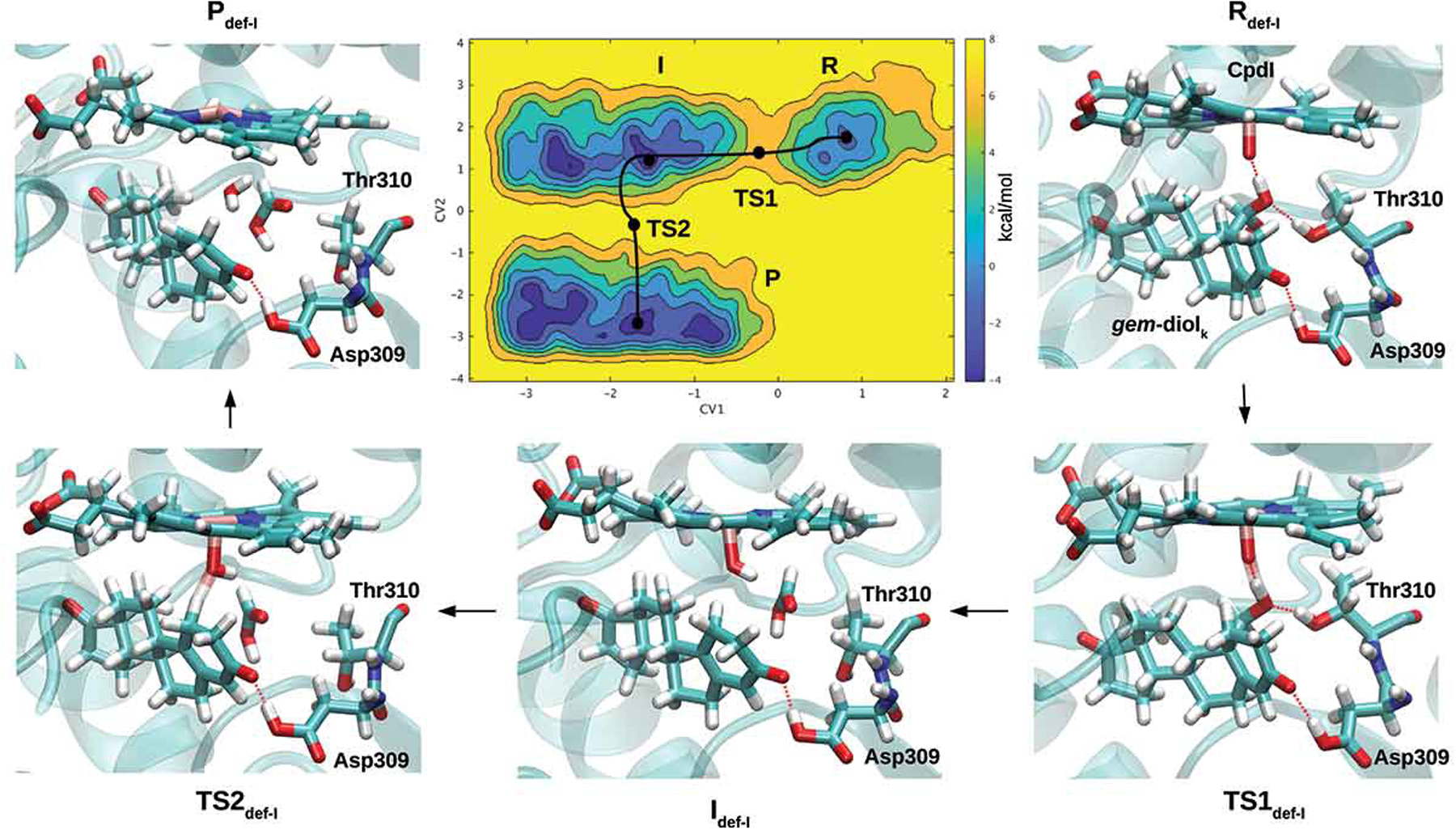
A) Free-energy surface for the aromatization (third catalytic) step, considering the enol form of androstenedione as a reagent [108]. Representative snapshots for the reagent (R), intermediate (I), product (P) and transition states (TSs) formed along the lowest energy path, are reported. Heme and the substrate are shown in a stick representation. Binding of B) letrozole and C) abiraterone to the Fe atom of CYP19A1 and CYP17A1, respectively, as predicted by QM/MM MD simulations [113] and X-ray crystallography (PDB 3RUK) [121]. Reproduced with permission from Ref [108], Copyright 2018 John Wiley & Sons.
