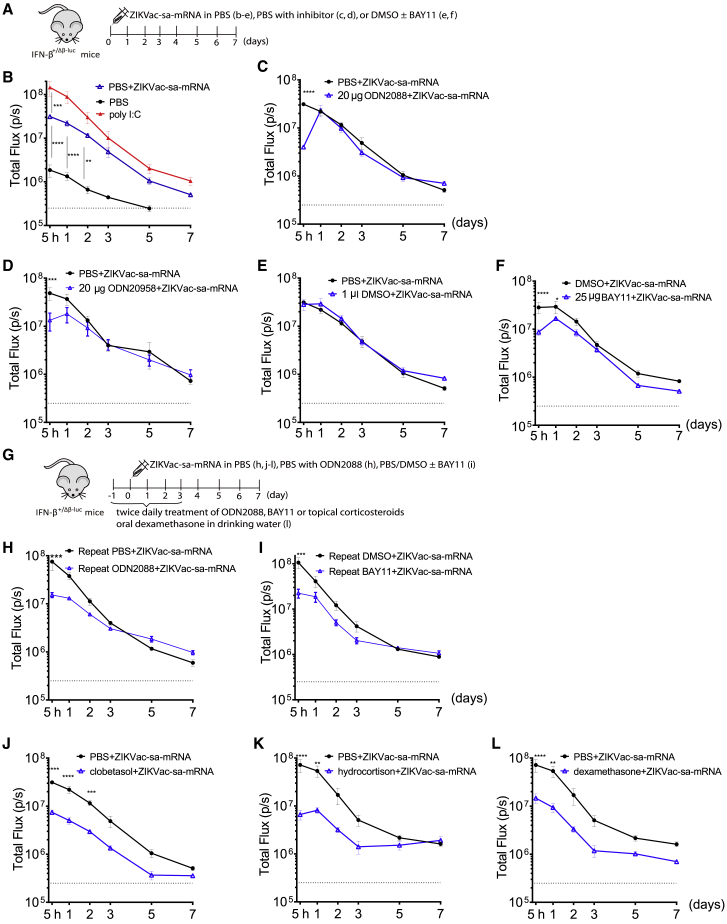
Figure 2.
Effect of innate immune inhibitors on the kinetics of the type I IFN response after intradermal electroporation of ZIKVac-sa-mRNA in IFN-β+/Δβ-luc reporter mice
(A) Inhibitors were mixed with the ZIKVac-sa-mRNA vaccine before administration to IFN-β+/Δβ-luc reporter mice. (B–F) The graphs represent the kinetics of the type I IFN response (y axis) after a single intradermal electroporation of the ZIKVac-sa-mRNA vaccine (1 μg in 50 μL), poly(I:C) (1 μg in 50 μL), or PBS control (B) and the capacity of the inhibitors ODN2088 (C), ODN20958 (D), and BAY11 (F) to temper type I IFN responses elicited by the ZIKVac-sa-mRNA. Because DMSO is needed to dissolve BAY11, the influence of DMSO was also studied (E). (G) The treatment schedule of repeated administration of inhibitors to IFN-β+/Δβ-luc reporter mice. (H and I) The effect of repeated ODN2088 or BAY11 administration; the injection site was injected intradermally with the inhibitors 5 h prior to ZIKVac-sa-mRNA administration. Subsequently, the ZIKVac-sa-mRNA vaccine was administered together with the inhibitors ODN2088 (H) or BAY11 (I). On the day of sa-mRNA administration, a second local injection of ODN2088 or BAY11 was given after 7 h. Local injection of the inhibitors continued twice daily until day 3. (J and K) In contrast to the other inhibitors, clobetasol propionate (J) and hydrocortisone (K) were applied topically (25 μg/1 cm2) 1 day prior to ZIKVac-sa-mRNA administration, and this was repeated twice daily until day 3. (L) Dexamethasone was administered orally in drinking water for 4 days starting on day −1. Each symbol represents the mean of four individual mice, and the error bars represent SEM. The PBS+ZIKVac-sa-mRNA blanc was repeated each time, except in (C) and (L), where we used the same blanc as in (A) and (K), respectively.