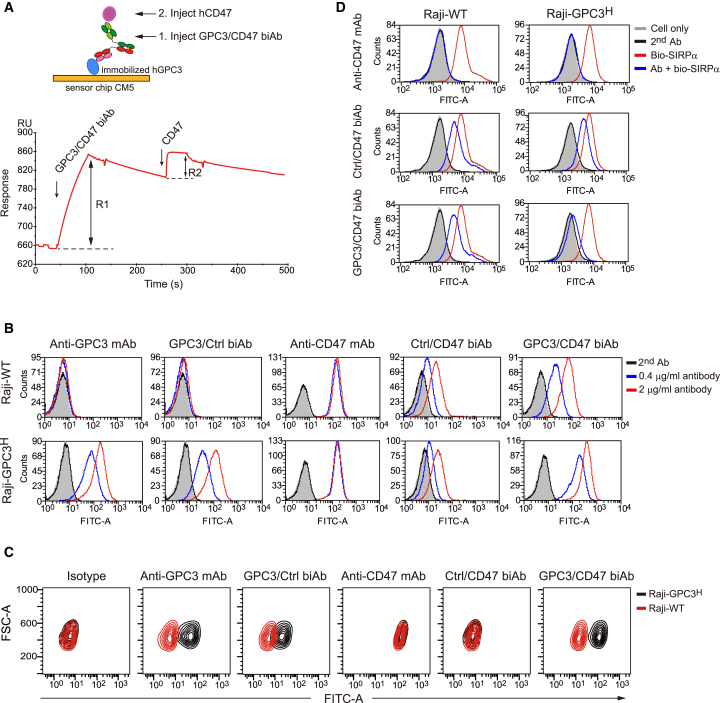
Figure 1.
Binding ability and selectivity analysis of the GPC3/CD47 biAb
(A) Binding curve of the GPC3/CD47 biAb with the GPC3 or CD47 antigens as measured by SPR-based bridge assays (depicted in the schematic). The blue and magenta circles, respectively, denote GPC3 and CD47. R1: binding response of the GPC3/CD47 biAb to the immobilized GPC3. R2: binding response of CD47 to the GPC3/CD47 biAb. (B) The binding ability of each antibody (Ab) to Raji-WT and Raji-GPC3H cells was detected by flow cytometry. (C) The binding selectivity of each antibody was measured by competitive flow cytometry. Raji-WT cells (red contour) mixed with Raji-GPC3H cells (black contour) at a ratio of 1:1 were incubated with 0.2 μg/mL of each antibody prior to staining with FITC-conjugated anti-human (h)IgG secondary antibody. The binding selectivity was determined based on the fluorescence intensity for FITC in each cell population. (D) GPC3/CD47 biAb competes with SIRPα for binding to Raji-GPC3H cells, exerting stronger competitive ability than Raji-WT. Raji-WT or Raji-GPC3H cells were incubated with 50 nM of biotinylated SIRPα-mFc fusion protein in the presence of anti-CD47 mAb, control (Ctrl)/CD47 biAb, and GPC3/CD47 biAb (10 μg/mL). The binding of SIRPα to each cell type was assessed based on the fluorescence intensity of FITC-conjugated streptavidin. See also Figure S1.