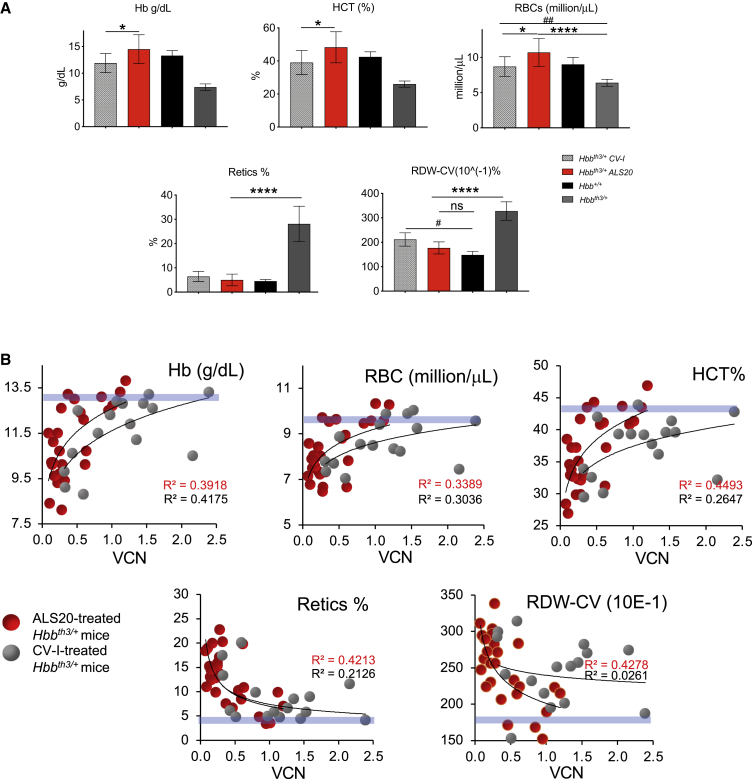
Figure 4.
Dose-response analysis for viral copy number and hemoglobin concentration and complete blood count assessment of experimental mice
(A) Hemoglobin (Hb) concentration (top left), hematocrit (HCT, %; top middle), red blood cell (RBC) count (top right), reticulocyte count (%; bottom left), and RBC distribution width coefficient of variation (RDW-CV; bottom right) in Hbb+/+ (n = 3, 100% donor chimerism), Hbbth3/+ (n = 22, 96.3% donor chimerism), and in Hbbth3/+ mice treated with ALS20-T87Q (VCN = 1 ± 0.1) or CV-I (VCN = 1 ± 0.2) (n = 9, 95.5% donor chimerism, and 5, 93% donor chimerism for ALS20 and CV-I-treated groups, respectively). Groups were tested by non-parametric ANOVA analyses. Asterisks indicate p values that refer to ALS20, while pound sign indicates p values that refer to CV-I. ∗ or #p ≤ 0.05; ##p ≤ 0.005; and ∗∗∗∗p < 0.0001. (B) Hb concentration (top left), HCT (%; top middle), RBC count (top right), reticulocyte count (%; bottom left), and RDW (bottom right) are plotted against the VCN in all ALS20- or CV-I-treated Hbbth3/+ mice. Blue horizontal bars represent values in wild-type Hbbt+/+ mice. R squared for each trendline are indicated in red for ALS20 and in black for CV-I.