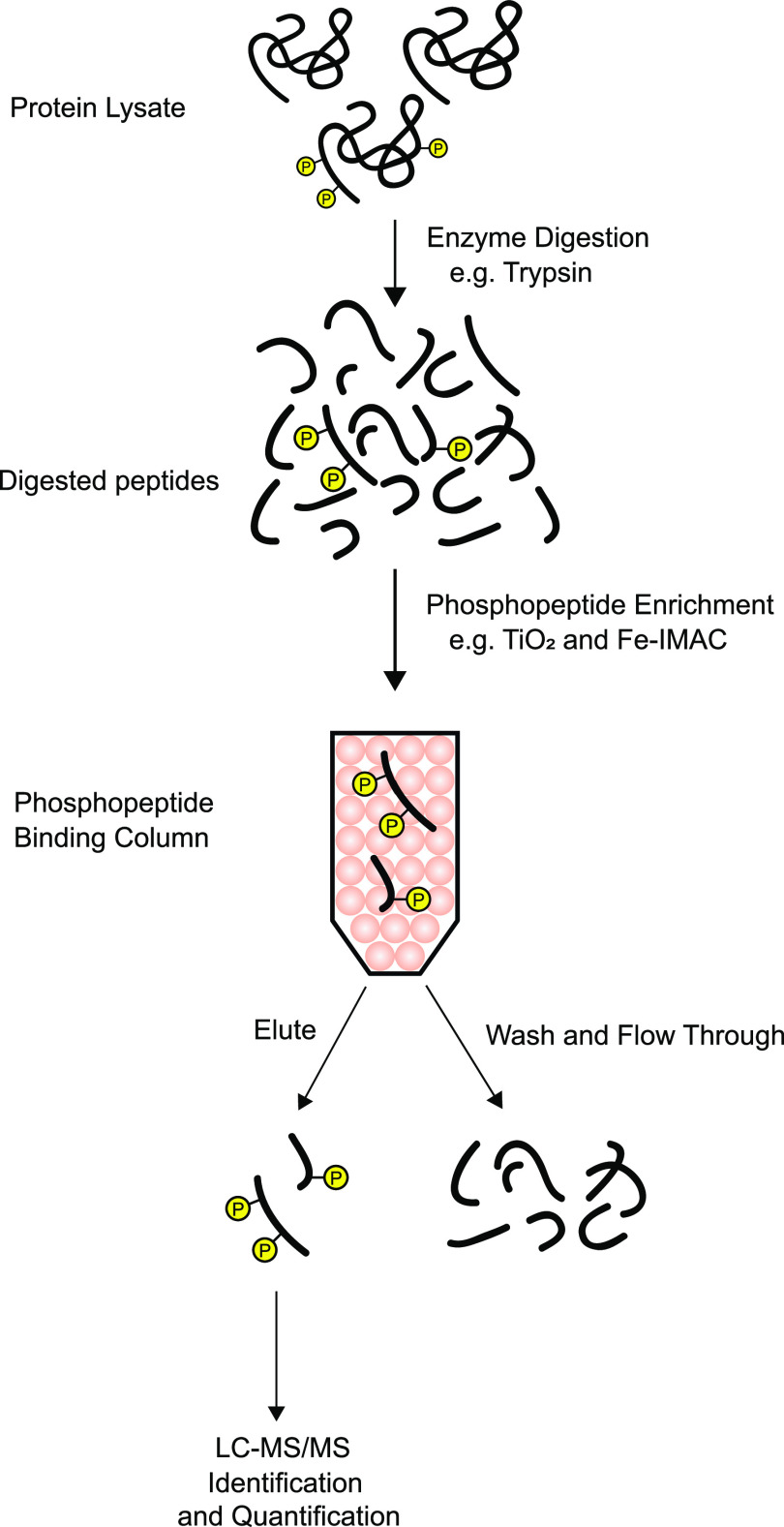
Fig. 2.
Simplified basic protocol for phosphoproteomic analysis. A protein sample is subjected to proteolysis with a purified, recombinant protease (typically trypsin). Phosphopeptides are enriched through use of ion chromatography, e.g., with TiO2 or Fe-IMAC columns, which select peptides with negative charges. The column eluate is subjected to LC-MS/MS analysis to identify and quantify phosphopeptides, often after additional fractionation (not shown). IMAC, immobilized metal affinity chromatography. Circled P indicates phosphorylation.