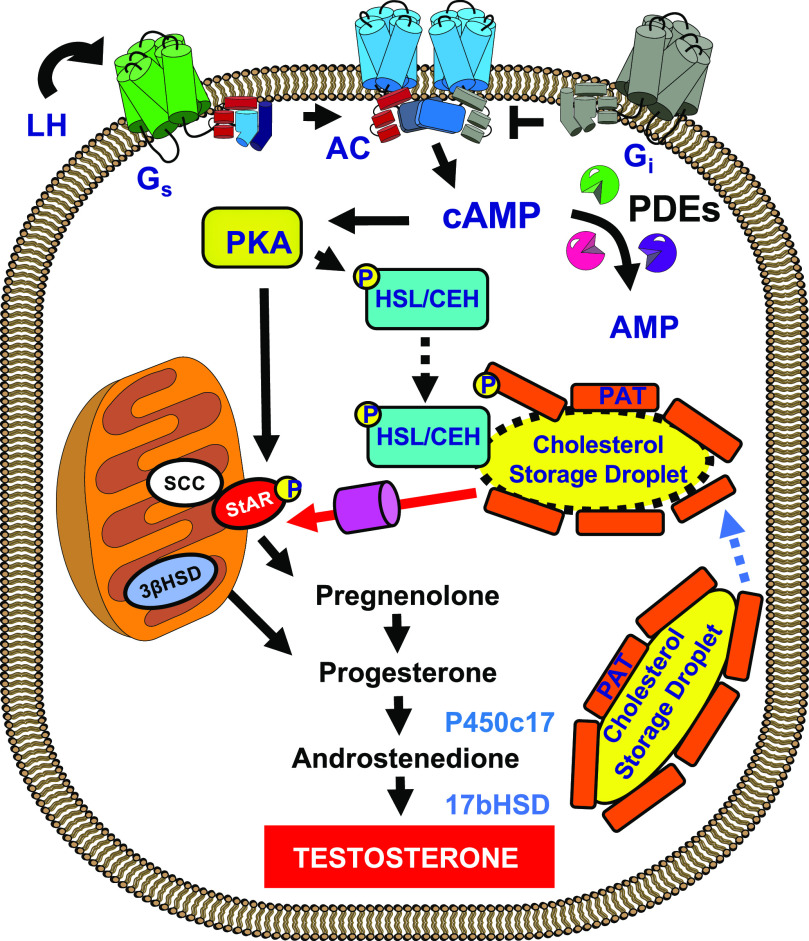
Fig. 1.
Model depicting parts of the classic cAMP-dependent, hormone-stimulated steroid-secretion pathway. In this case, the hormone is luteinizing hormone (LH) binding to the LH receptor. Receptor activation causes a G-protein–dependent activation of cAMP synthesis. The cAMP diffuses to many different compartments in the cell, where it interacts with PKA. The cAMP concentration is also modulated by several different PDEs. Once activated, PKA will phosphorylate and activate several specific proteins in the steroidogenic pathway. One of the best studied is hormone-sensitive lipase (HSL/Lipe), also originally known as CEH, which, acting with PAT proteins, such as perilipin (Plin), stimulates the production of free cholesterol from cholesteryl esters stored in lipid droplets. Another important PKA target is StAR, which assists cholesterol entry into the mitochondria, where much of the conversion of cholesterol to hormone occurs. On a longer time scale, PKA also stimulates the synthesis of several other steroidogenic enzymes, including P450 steroid 17 α-monooxygenase (c17) and 17β-hydroxysteroid dehydrogenase (17b-HSD). Many other enzymes and steps are required for synthesis and may be regulated but are not depicted in this classic model. AC, adenylyl cyclase; SCC, Side Chain Cleavage (Also referred to as P450scc); PAT, acronym for perilipin.