Figure 2. Neutralization of SARS-CoV-2 viral variants by convalescent human serum in Vero-hACE2 TMPRSS2 cells.
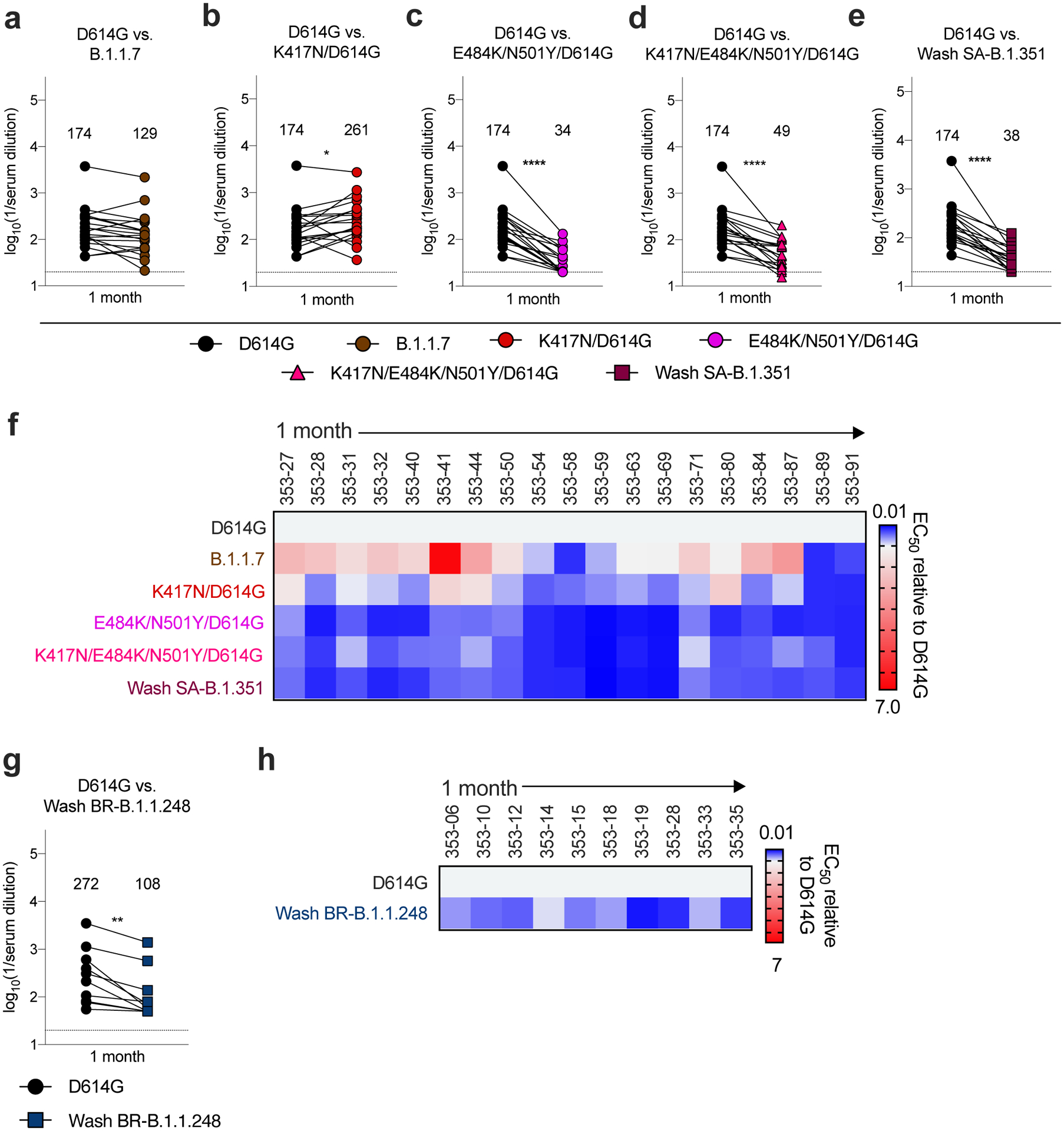
a-e, Paired analysis of neutralizing activity by convalescent human sera (n = 19) obtained approximately 1 month after mild SARS-CoV-2 infection against WA1/2020 D614G and variant viruses in Vero-hACE2-TMPRSS2 cells: (a) B.1.1.7, (b) K417N/D614G, (c) E484K/N501Y/D614G, (d) K417N/E484K/N501Y/D614G, or (e) Wash SA-B.1.351. g. Paired analysis of neutralizing activity by a separate convalescent human sera cohort (n = 10) obtained approximately 1 month after mild SARS-CoV-2 infection against WA1/2020 D614G and Wash BR-B.1.1.248 in Vero-hACE2-TMPRSS2 cells. a-e and g. Results are from one experiment performed in duplicate. Geometric mean neutralization titers (GMT) are shown above each graph. Dotted line represents the limit of detection of the assay. Two-tailed Wilcoxon matched-pairs signed rank test: D614G vs. B.1.1.7, P = 0.0546; D614G vs. K417N/D614G, P = 0.0361; D614G vs. E484K/N501Y/D614G, P < 0.0001; D614G vs. K417N/E484K/N501Y/D614G, P < 0.0001; D614G vs. Wash SA-B.1.351, P < 0.0001; D614G vs. Wash BR-B.1.1.248, P = 0.0020. f, h Heat maps of the relative neutralizing activity of sera from individual convalescent subjects against indicated SARS-CoV-2 viruses compared to recombinant WA1/2020 D614G. Blue, reduction; red, increase.
