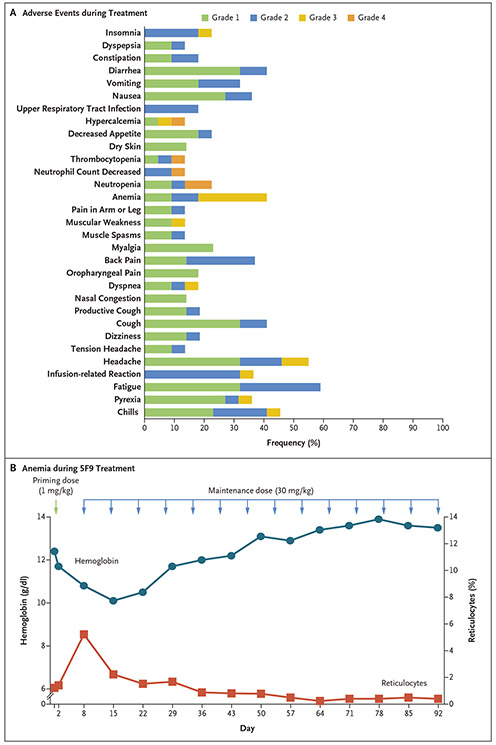
Figure 1. Adverse Events Due to Hu5F9-G4 (5F9), Rituximab, or Both and On-Target Anemia Effect of 5F9.
Panel A shows the adverse events that occurred in at least 10% of the patients during treatment. Panel B shows the levels of hemoglobin and reticulocytes over time in a representative patient (with diffuse large B-cell lymphoma) during the study. The priming dose of 1 mg of 5F9 per kilogram of body weight was received on day 1 (green arrow). The patient received maintenance doses of 30 mg of 5F9 per kilogram (blue arrows).