Table 2.
Substrate Scope for the CuH-Catalyzed Hydrocarboxylation of Allenesa
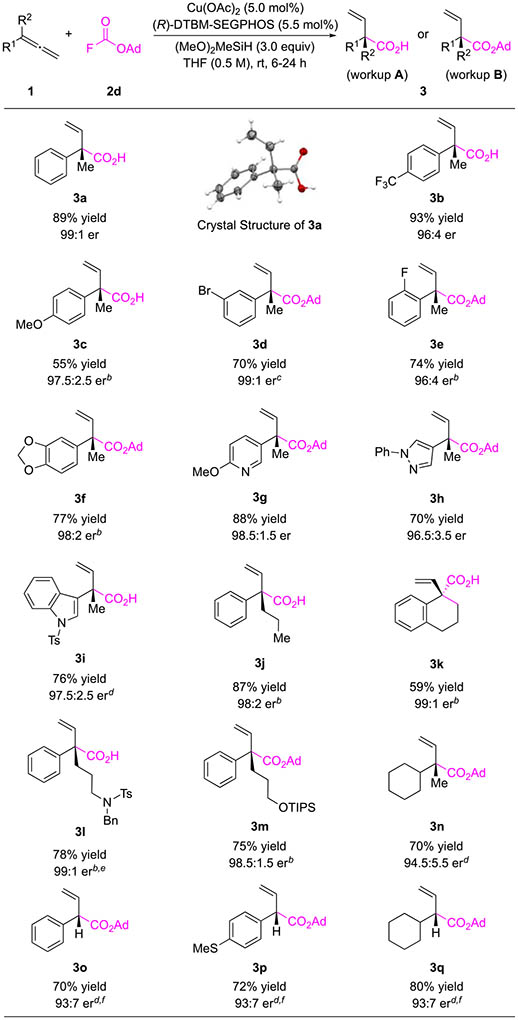
|
Conditions: 0.50 mmol 2d (1.0 equiv), 1 (1.2 equiv), copper(II) acetate (5.0 mol %), L2 (5.5 mol %), dimethoxy(methyl)silane (3.0 equiv) in THF (0.5 M); workup A: NH4F/MeOH workup followed by hydrolysis using TFA; workup B: NH4F/MeOH workup; yields refer to average isolated yields of two runs; see the Supporting Information for details.
Reaction was carried out at 40 °C.
Reaction was carried out at 30 °C.
L3 was used as the ligand instead.
1 (1.1 equiv) was used. fReaction was carried out at 0 °C in 1,2-dimethoxyethane (DME, 1.0 mL).
