Table 2.
Amination of Aryl Triflates using P1a
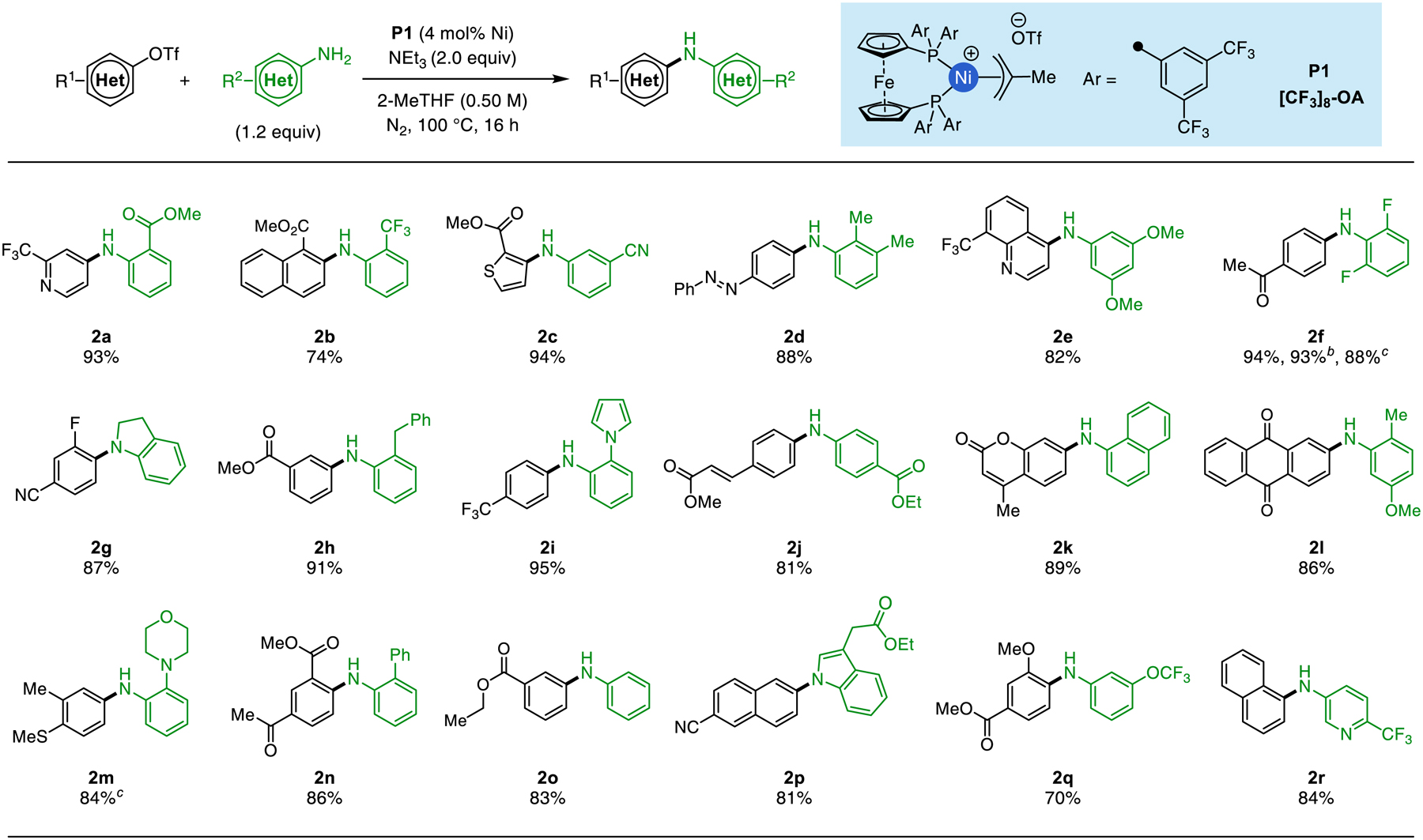
|
Isolated yields are reported as the average of two runs. Unless noted, standard reaction conditions: aryl triflate (1.0 mmol), aryl amine (1.2 mmol), triethylamine (2.0 mmol), P1(0.04 mmol, 4% Ni), 2-MeTHF (2.0 mL, 0.5 M), 100 °C for 16 h.
Single reaction performed without stirring.
5.0 mmol scale reaction using 2.0% Ni.
1.5 equiv of aryl amine was used.
