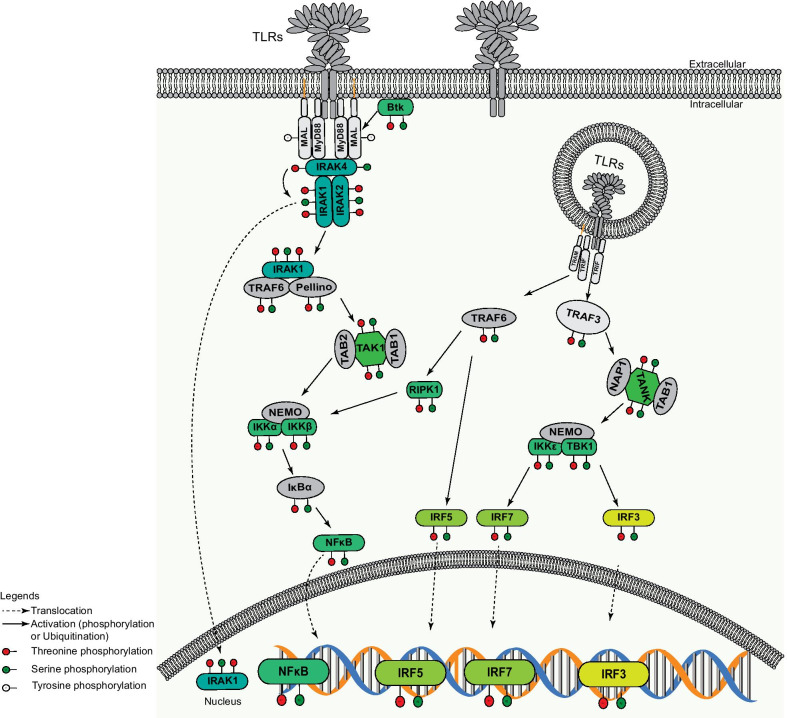
Fig. 1.
Phosphorylation events in TLR signaling. TLRs recognize their respective ligands at the cell surface or endosomes, leading to recruitment of MyD88. A receptor complex (Myddosome or Triffosome) is formed with IRAK4 and IRAK1 (and IRAK2 in the late phase). IRAK4 phosphorylates IRAK1, and the TRAF6-IRAK1 complex dissociates from MyD88. IRAK1 undergoes autophosphorylation and is degraded by the proteasome upon Pellino-1-mediated polyubiquitination. Pellino-1 and TRAF6 recruit TAK1-TAB2-TAB1 complex and IKKα/β-NEMO Complex. TAK1 is autophosphorylated and phosphorylates IKKα/β-NEMO Complex. Subsequently, IKKα/β phosphorylates IκBα bound to NF-κB p65. The latter is released and translocates to the nucleus. In the TRIF dependent pathway, TRAF6 recruits either RIPK1 and activates the IKK-IκBα-NF-κB p65 axis, or IRF5. On the other hand, TRAF3 recruits NAP1-TANK-TAB1 and NEMO-IKKε-TBK1 complexes, upon which TANK phosphorylates and activates IKKε and TBK1. Both kinases trigger IRF3 or IRF7-mediated type-I interferon production. Phosphorylated tyrosine, serine and threonine residues are represented by white, green, and circles red, respectively. Activation (ubiquitination or phosphorylation) and translocation are indicated by plain or dashed arrows, respectively