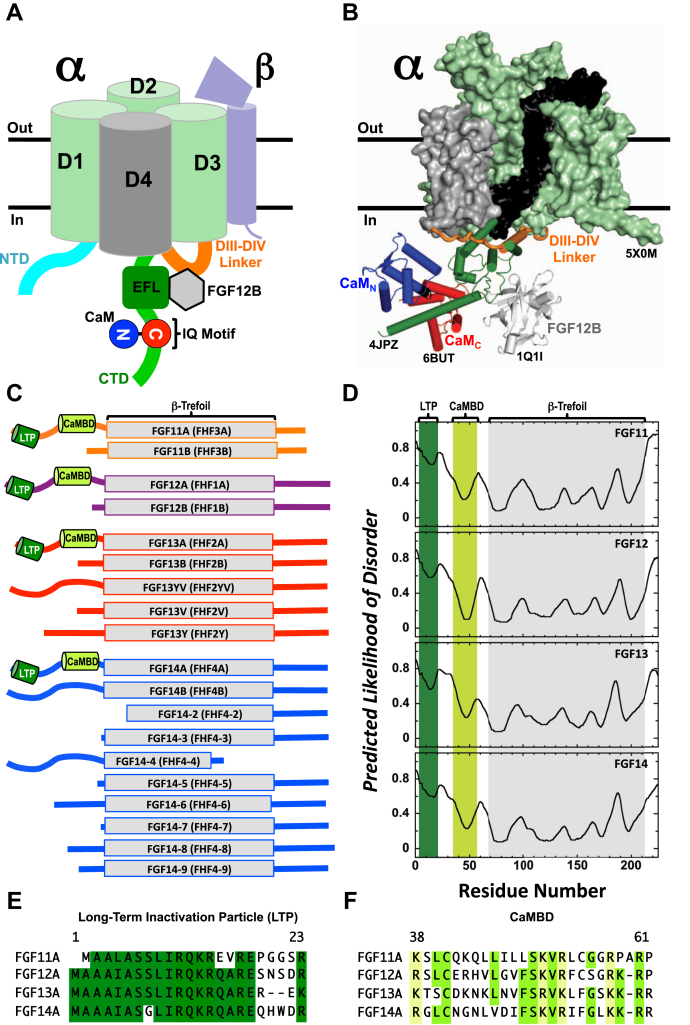
Figure 1.
Architecture of NaV, CaM, and FGFs.A, schematic of human NaV. The α-subunit N-terminal domain (NTD, cyan), transmembrane domains DI-DIII (pale green) and DIV (gray), the linker connecting DIII to DIV (orange), and C-terminal domain (CTD, green) with 4-helix bundle EFL (dark green) that binds FGFs (light gray), and IQ motif that binds CaM (CaMN/blue, CaMC/red) are shown. Auxiliary β-subunit that contains a transmembrane helix and extracellular domain is lavender. B, model of apo CaM and FGF12B bound to NaV. Model is comprised of NaVPAS (5X0M, DI-DIII/pale green surface, DIV voltage-sensing domain/gray surface, DIV pore domain/black surface, DIII-DIV linker/orange cartoon), the NaV1.5CTD (4DCK, forest green), the apo CaM+NaV1.2IQp ensemble (6BUT, CaMN/blue, CaMC/red), and FGF12B (1Q1U, gray). The NaV1.5CTD was aligned to NaVPAS EFL (a.a. 1426–1521), the apo CaM+NaV1.2IQp ensemble was aligned with 4DCK via CaM a.a. 101–112 and 117–128, and FGF12B was aligned with 4DCK using FGF13B a.a. 11–158. For simplicity NaV1.2IQp in 6BUT and CaM FGF13B in 4DCK are not shown. C, schematics of FGF11 (orange), FGF12A (purple), FGF13A (red), and FGF14 (blue) splice variants. The β-trefoil core is shown as a rectangle, and the long-term inactivation particle (LTP, green) and putative CaM-binding domain (CaMBD, limon) are shown as cylinders. D, predicted likelihood of disorder of A-type FGF isoform sequences. The minima shaded green and limon correspond to the LTP and CaMBD sequences, respectively. The folded β-trefoil core is shaded gray. E and F, sequence alignments of the A-type FGF LTP (E) and CaMBD (F). Positions that are conserved in at least three of the four FGF isoforms are shaded (LTP/green, CaMBD/limon). Positions in the CaMBD (F) that contain a basic K or R in all four isoforms are shaded in pale yellow. Alignments were made with COBALT (108).