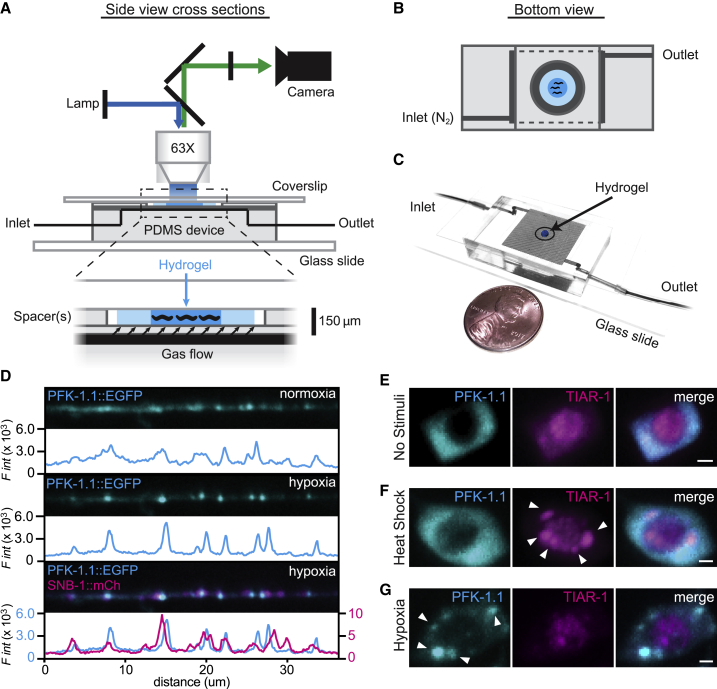
Figure 2.
Hybrid microfluidic-hydrogel device enables high-resolution imaging of PFK-1.1 localization and clustering dynamics during transient hypoxia. (A) A schematic of the hybrid microfluidic-hydrogel device used to induce transient hypoxic conditions in C. elegans while imaging PFK-1.1 continuously and at high resolution. Zoom-in (below) shows micron-scale device geometries for animal immobilization, gas delivery, and imaging. (B) A bottom view schematic of the device shows geometry of inlet and outlet channels connected to the gas arena (dark gray area). Animals are immobilized in hydrogel (dark blue) surrounded by buffer (light blue) and sealed by a circular, hole-punched PDMS spacer. Oxygen conditions experienced by the animals are controlled by inlet gas flow. (C) A picture of the microfluidic device, size comparable to a penny, with inlet and outlet tubing connected. (D) PFK-1.1::EGFP (cyan) in GABAergic neuron under normoxia (top panels) and after 10 min of transient hypoxia (middle panels). Coexpression with synaptobrevin-1/SNB-1::mCh (magenta, lower panel) shows that PFK-1.1 relocalized into clusters near synaptic sites upon transient hypoxia. Corresponding fluorescence intensity for each image is shown immediately below the corresponding image, with colors matching traced protein distribution in the image. (E) PFK-1.1::mRuby-3 (cyan) and TIAR-1::EGFP (magenta) in the cell body of neurons with no stimuli (normoxic conditions and 22°C). (F) Heat shock (37°C for 1 h) leads to formation of stress granules, as observed with TIAR-1 aggregates (arrowheads). PFK-1.1 does not form clusters under this condition. (G) Under transient hypoxic conditions (25–45 min), PFK-1.1 forms clusters in the cell body (arrowheads) that do not colocalize with TIAR-1. All scale bars, 1 μm.